Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study
- PMID: 31222951
- PMCID: PMC7079122
- DOI: 10.1111/1744-9987.12888
Intermittent Oral Dosing of Roxadustat in Peritoneal Dialysis Chronic Kidney Disease Patients with Anemia: A Randomized, Phase 3, Multicenter, Open-Label Study
Abstract
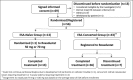
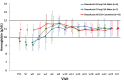
Roxadustat is an oral hypoxia-inducible factor prolyl hydroxylase inhibitor developed to treat anemia in chronic kidney disease (CKD) patients. This Phase 3, randomized, open-label, 24-week study investigated the efficacy and safety of roxadustat in Japanese CKD patients with anemia on peritoneal dialysis (PD) who were previously treated or not treated with erythropoiesis stimulating agents (ESAs). Patients not previously receiving ESA (ESA-Naïve group) were randomized to roxadustat at a starting dose of 50 or 70 mg three times weekly; patients previously receiving ESA (ESA-Converted group) switched from ESA to roxadustat 70 or 100 mg three times weekly depending on the prior ESA dose. Outcomes included maintenance rate of average hemoglobin (Hb) level within 10-12 g/dL at weeks 18-24, cumulative response rate at end of treatment (Hb thresholds, 10.0 g/dL or 10.5 g/dL; Hb increase, ≥1.0 g/dL), and average Hb levels at weeks 18-24. Safety was assessed by occurrence of treatment-emergent adverse events (TEAEs). Fifty-six patients were enrolled (ESA-Naïve, n = 13; ESA-Converted, n = 43). Maintenance rates (weeks 18-24) were 92.3% (95% CI: 64.0-99.8; ESA-Naïve) and 74.4% (95% CI: 58.8-86.5; ESA-Converted). Cumulative response rate was 100.0% in the ESA-Naïve group. Average Hb levels (weeks 18-24) were 11.05 g/dL (95% CI: 10.67-11.42; ESA-Naïve) and 10.93 g/dL (95% CI: 10.73-11.13; ESA-Converted). Common TEAEs included nasopharyngitis and back pain. Roxadustat was well tolerated and effective in maintaining target Hb levels in CKD patients on PD who were previously treated or not treated with ESA.
Keywords: Anemia; Chronic kidney disease; Clinical trial; Peritoneal dialysis; Roxadustat.
© 2019 The Authors. Therapeutic Apheresis and Dialysis published by John Wiley & Sons Australia, Ltd on behalf of International Society for Apheresis, Japanese Society for Apheresis, and Japanese Society for Dialysis Therapy.
Conflict of interest statement
Tadao Akizawa reports personal fees from Astellas, Bayer Yakuhin Ltd., GlaxoSmithKline, JT Pharmaceuticals, Kissei Pharmaceutical, Kyowa Hakko Kirin, and Chugai Pharmaceutical during the conduct of the study and reports personal fees from Ono Pharmaceutical, Fuso Pharmaceutical Industries, Nipro Corporation, and Torii Pharmaceutical outside of the submitted work. Tetsuro Otsuka and Mai Ueno are employees of Astellas Pharma and both own Astellas stock. Michael Reusch is an employee of Astellas Pharma Europe B.V.
Figures
References
-
- Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting hypoxia‐inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol 2017;45:187–99. - PubMed
-
- Krediet RT, Abrahams AC, de Fijter CWH et al. The truth on current peritoneal dialysis: state of the art. Neth J Med 2017;75:179–89. - PubMed
-
- Li PK, Chow KM, Van de Luijtgaarden MW et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 2017;13:90–103. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

