Plasmonic imaging of subcellular electromechanical deformation in mammalian cells
- PMID: 31222988
- PMCID: PMC6586072
- DOI: 10.1117/1.JBO.24.6.066007
Plasmonic imaging of subcellular electromechanical deformation in mammalian cells
Abstract
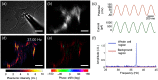
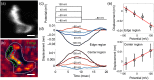
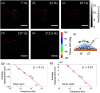
A membrane potential change in cells is accompanied with mechanical deformation. This electromechanical response can play a significant role in regulating action potential in neurons and in controlling voltage-gated ion channels. However, measuring this subtle deformation in mammalian cells has been a difficult task. We show a plasmonic imaging method to image mechanical deformation in single cells upon a change in the membrane potential. Using this method, we have studied the electromechanical response in mammalian cells and have observed the local deformation within the cells that are associated with cell-substrate interactions. By analyzing frequency dependence of the response, we have further examined the electromechanical deformation in terms of mechanical properties of cytoplasm and cytoskeleton. We demonstrate a plasmonic imaging approach to quantify the electromechanical responses of single mammalian cells and determine local variability related to cell-substrate interactions.
Keywords: cell electromechanics; electromechanical coupling; electromechanical deformation; plasmonic imaging.
Figures


Similar articles
-
Voltage-dependent membrane displacements measured by atomic force microscopy.J Gen Physiol. 1998 Jan;111(1):65-74. doi: 10.1085/jgp.111.1.65. J Gen Physiol. 1998. PMID: 9417135 Free PMC article.
-
Electro-deformation spectroscopy: A unified method for simultaneous electrical and mechanical characterization of single cells.Acta Biomater. 2025 Jan 15;192:119-127. doi: 10.1016/j.actbio.2024.12.012. Epub 2024 Dec 5. Acta Biomater. 2025. PMID: 39644941
-
Interferometric plasmonic imaging and detection of single exosomes.Proc Natl Acad Sci U S A. 2018 Oct 9;115(41):10275-10280. doi: 10.1073/pnas.1804548115. Epub 2018 Sep 24. Proc Natl Acad Sci U S A. 2018. PMID: 30249664 Free PMC article.
-
Analyzing Protein Clusters on the Plasma Membrane: Application of Spatial Statistical Analysis Methods on Super-Resolution Microscopy Images.Adv Anat Embryol Cell Biol. 2016;219:95-122. doi: 10.1007/978-3-319-28549-8_4. Adv Anat Embryol Cell Biol. 2016. PMID: 27207364 Review.
-
Intermolecular interactions with/within cell membranes and the trinity of membrane potentials: kinetics and imaging.Biochem Soc Trans. 2003 Oct;31(Pt 5):990-6. doi: 10.1042/bst0310990. Biochem Soc Trans. 2003. PMID: 14505466 Review.
Cited by
-
On the Coupling between Mechanical Properties and Electrostatics in Biological Membranes.Membranes (Basel). 2021 Jun 28;11(7):478. doi: 10.3390/membranes11070478. Membranes (Basel). 2021. PMID: 34203412 Free PMC article. Review.
-
Optical Electrophysiology: Toward the Goal of Label-Free Voltage Imaging.J Am Chem Soc. 2021 Jul 21;143(28):10482-10499. doi: 10.1021/jacs.1c02960. Epub 2021 Jun 30. J Am Chem Soc. 2021. PMID: 34191488 Free PMC article.
-
High-speed interferometric imaging reveals dynamics of neuronal deformation during the action potential.Proc Natl Acad Sci U S A. 2020 May 12;117(19):10278-10285. doi: 10.1073/pnas.1920039117. Epub 2020 Apr 27. Proc Natl Acad Sci U S A. 2020. PMID: 32341158 Free PMC article.
-
Deep Learning Enhanced Label-Free Action Potential Detection Using Plasmonic-Based Electrochemical Impedance Microscopy.Anal Chem. 2024 Jul 16;96(28):11299-11308. doi: 10.1021/acs.analchem.4c01179. Epub 2024 Jul 2. Anal Chem. 2024. PMID: 38953225 Free PMC article.
-
Reply to Farrell: Experimental evidence is the ultimate judge for model assumptions.Proc Natl Acad Sci U S A. 2020 Oct 27;117(43):26574-26575. doi: 10.1073/pnas.2017702117. Epub 2020 Oct 13. Proc Natl Acad Sci U S A. 2020. PMID: 33051290 Free PMC article. No abstract available.

