Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour
- PMID: 31223140
- PMCID: PMC6565756
- DOI: 10.1016/j.ynpai.2019.100032
Cathepsin S acts via protease-activated receptor 2 to activate sensory neurons and induce itch-like behaviour
Abstract
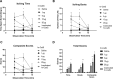
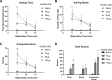
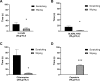
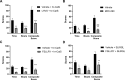
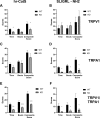
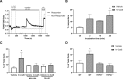
Chronic itch is a debilitating condition characterised by excessive scratching and is a symptom frequently reported in skin diseases such as atopic dermatitis. It has been proposed that release of the cysteine protease Cathepsin S (CatS) from skin keratinocytes or immune cells resident in or infiltrating the skin could act as a pruritogen in chronic itch conditions. CatS is known to activate protease-activated receptor 2 (PAR2). We therefore hypothesised that enzymatic activation of neuronally expressed PAR2 by CatS was responsible for activation of sensory neurons and transmission of itch signals. Intradermally-injected human recombinant (hr)-CatS or the PAR2 agonist, SLIGRL-NH2 behaved as pruritogens by causing scratching behaviour in mice. Hr-CatS-induced scratching behaviour was prevented by CatS inhibitors and PAR2 antagonists and reduced by 50% in TRPV1-/- mice compared with wild-type mice, whilst no significant reduction in scratching behaviour was observed in TRPA1-/- mice. Cultured dorsal root ganglion (DRG) cells showed an increase in [Ca2+]i following incubation with hr-CatS, and the percentage of neurons that responded to hr-CatS decreased in the presence of a PAR2 antagonist or in cultures of neurons from TRPV1-/- mice. Taken together, our results indicate CatS acts as a pruritogen via PAR2 activation in TRPV1-expressing sensory neurons.
Keywords: Itch; Proteases; Sensory neurons.
Figures
Similar articles
-
Targeting Transient Receptor Potential (TRP) Channels, Mas-Related G-Protein-Coupled Receptors (Mrgprs), and Protease-Activated Receptors (PARs) to Relieve Itch.Pharmaceuticals (Basel). 2023 Dec 8;16(12):1707. doi: 10.3390/ph16121707. Pharmaceuticals (Basel). 2023. PMID: 38139833 Free PMC article. Review.
-
FSLLRY-NH2, a protease-activated receptor 2 (PAR2) antagonist, activates mas-related G protein-coupled receptor C11 (MrgprC11) to induce scratching behaviors in mice.Life Sci. 2023 Jul 15;325:121786. doi: 10.1016/j.lfs.2023.121786. Epub 2023 May 16. Life Sci. 2023. PMID: 37201698
-
A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1.J Allergy Clin Immunol. 2014 Feb;133(2):448-60. doi: 10.1016/j.jaci.2013.10.048. Epub 2013 Dec 25. J Allergy Clin Immunol. 2014. PMID: 24373353 Free PMC article.
-
Genetic priming of sensory neurons in mice that overexpress PAR2 enhances allergen responsiveness.Proc Natl Acad Sci U S A. 2021 Feb 23;118(8):e2021386118. doi: 10.1073/pnas.2021386118. Proc Natl Acad Sci U S A. 2021. PMID: 33602818 Free PMC article.
-
Mediators of Chronic Pruritus in Atopic Dermatitis: Getting the Itch Out?Clin Rev Allergy Immunol. 2016 Dec;51(3):263-292. doi: 10.1007/s12016-015-8488-5. Clin Rev Allergy Immunol. 2016. PMID: 25931325 Review.
Cited by
-
Targeting Transient Receptor Potential (TRP) Channels, Mas-Related G-Protein-Coupled Receptors (Mrgprs), and Protease-Activated Receptors (PARs) to Relieve Itch.Pharmaceuticals (Basel). 2023 Dec 8;16(12):1707. doi: 10.3390/ph16121707. Pharmaceuticals (Basel). 2023. PMID: 38139833 Free PMC article. Review.
-
Pruritogens in pemphigoid diseases: Possible therapeutic targets for a burdensome symptom.J Dermatol. 2023 Feb;50(2):150-161. doi: 10.1111/1346-8138.16652. Epub 2022 Dec 7. J Dermatol. 2023. PMID: 36477831 Free PMC article. Review.
-
Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation.Int J Mol Sci. 2021 Nov 16;22(22):12365. doi: 10.3390/ijms222212365. Int J Mol Sci. 2021. PMID: 34830245 Free PMC article. Review.
-
Calcium Increase and Substance P Release Induced by the Neurotoxin Brevetoxin-1 in Sensory Neurons: Involvement of PAR2 Activation through Both Cathepsin S and Canonical Signaling.Cells. 2020 Dec 17;9(12):2704. doi: 10.3390/cells9122704. Cells. 2020. PMID: 33348659 Free PMC article.
-
Par2-mediated responses in inflammation and regeneration: choosing between repair and damage.Inflamm Regen. 2024 May 30;44(1):26. doi: 10.1186/s41232-024-00338-1. Inflamm Regen. 2024. PMID: 38816842 Free PMC article. Review.
References
-
- Andersen H.H., Elberling J., Sølvsten H., Yosipovitch G., Arendt-Nielsen L. Nonhistaminergic and mechanical itch sensitization in atopic dermatitis. Pain. 2017 - PubMed
-
- Atoyan R., Shander D., Botchkareva N.V. Non-neuronal expression of transient receptor potential type A1 (TRPA1) in human skin. J. Invest. Dermatol. 2009 - PubMed
-
- Barclay J., Clark A.K., Ganju P., Gentry C., Patel S., Wotherspoon G., Buxton F., Song C., Ullah J., Winter J., Fox A., Bevan S., Malcangio M. Role of the cysteine protease cathepsin S in neuropathic hyperalgesia. Pain. 2007 - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

