Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus
- PMID: 31223141
- PMCID: PMC6565752
- DOI: 10.1016/j.ynpai.2019.100031
Sex differences in the expression of calcitonin gene-related peptide receptor components in the spinal trigeminal nucleus
Abstract
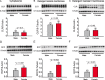
Background and purpose: Calcitonin gene-related peptide (CGRP) plays an important role in migraine pathophysiology. CGRP acts primarily by activating a receptor composed of 3 proteins: calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and receptor component protein (RCP). We tested the hypothesis that sex differences exist in protein levels of two key components of this CGRP receptor: CLR and RCP.
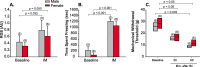
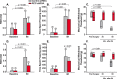
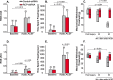
Methods: We used specific antibodies to assess baseline protein levels of CLR and RCP in the spinal trigeminal nucleus caudalis (SpVc) and upper cervical spinal cord of both male and female rats. We also tested if manipulations that knock-down the expression of RCP in SpVc, using locally-mediated gene transfer of short hairpin RNA (shRNA), ameliorate pain in an animal model of intracranial migraine-like pain induced by chemical noxious stimulation of the meninges. To assess pain, we used tests of ongoing pain (rat face grimace test and freezing behavior) and tests of facial mechanical hypersensitivity and allodynia.
Results: There was no difference in CLR levels between male and female animals (p > 0.11) in SpVc and the upper cervical cord. However, female animals exhibited greater baseline levels of RCP (up to 3-fold higher) compared to males (p < 0.002). The knock-down of RCP expression in SpVc attenuated mechanical facial allodynia induced by chemical noxious stimulation of the meninges, but had little effect on ongoing pain behaviors in female and male animals.
Conclusions: RCP is an integral component of the CGRP receptor and may play a key role in mediating CGRP induced central sensitization after noxious stimulation of the meninges. RCP expression in the SpVc and upper cervical cord is sexually dimorphic, with higher levels of expression in females. This dimorphism may be related to the increased incidence of migraines in females-a hypothesis that should be tested in the future.
Keywords: ACSF, artificial cerebrospinal fluid; Allodynia; CGRP; CGRP, calcitonin gene-related peptide; CLR, calcitonin receptor-like protein; Headache; IM, inflammatory mediators; Meninges; Migraine; RAMP1, receptor activity-modifying protein 1; RCP, receptor component protein; SpVc, spinal trigeminal nucleus caudalis; Trigeminal; shRNA, short hairpin RNA.
Figures

Similar articles
-
Sex differences in behavior and expression of CGRP-related genes in a rodent model of chronic migraine.Headache. 2011 May;51(5):674-92. doi: 10.1111/j.1526-4610.2011.01882.x. Headache. 2011. PMID: 21521205 Free PMC article.
-
Calcitonin receptor-like receptor (CLR), receptor activity-modifying protein 1 (RAMP1), and calcitonin gene-related peptide (CGRP) immunoreactivity in the rat trigeminovascular system: differences between peripheral and central CGRP receptor distribution.J Comp Neurol. 2008 Mar 20;507(3):1277-99. doi: 10.1002/cne.21607. J Comp Neurol. 2008. PMID: 18186028
-
Calcitonin gene-related peptide (CGRP) and its receptor components in human and rat spinal trigeminal nucleus and spinal cord at C1-level.BMC Neurosci. 2011 Nov 10;12:112. doi: 10.1186/1471-2202-12-112. BMC Neurosci. 2011. PMID: 22074408 Free PMC article.
-
CGRP receptor antagonism and migraine therapy.Curr Protein Pept Sci. 2013 Aug;14(5):386-92. doi: 10.2174/13892037113149990055. Curr Protein Pept Sci. 2013. PMID: 23745702 Review.
-
Neuropeptide effects in the trigeminal system: pathophysiology and clinical relevance in migraine.Keio J Med. 2011;60(3):82-9. doi: 10.2302/kjm.60.82. Keio J Med. 2011. PMID: 21979827 Review.
Cited by
-
Inhibiting endocytosis in CGRP+ nociceptors attenuates inflammatory pain-like behavior.Nat Commun. 2021 Oct 4;12(1):5812. doi: 10.1038/s41467-021-26100-6. Nat Commun. 2021. PMID: 34608164 Free PMC article.
-
CGRP physiology, pharmacology, and therapeutic targets: migraine and beyond.Physiol Rev. 2023 Apr 1;103(2):1565-1644. doi: 10.1152/physrev.00059.2021. Epub 2022 Dec 1. Physiol Rev. 2023. PMID: 36454715 Free PMC article. Review.
-
Serum Calcitonin Gene-Related Peptide and Receptor Protein Levels in Patients With Fibromyalgia Syndrome: A Cross-Sectional Study.Arch Rheumatol. 2020 Feb 7;35(4):463-467. doi: 10.46497/ArchRheumatol.2020.7783. eCollection 2020 Dec. Arch Rheumatol. 2020. PMID: 33758802 Free PMC article.
-
Sex Differences in CGRP Regulation and Function in the Amygdala in a Rat Model of Neuropathic Pain.Front Mol Neurosci. 2022 Jun 3;15:928587. doi: 10.3389/fnmol.2022.928587. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35726298 Free PMC article.
-
Migraine in men.J Headache Pain. 2025 Jan 3;26(1):3. doi: 10.1186/s10194-024-01936-7. J Headache Pain. 2025. PMID: 39754046 Free PMC article. Review.
References
-
- Agosti R. Migraine burden of disease: from the patient’s experience to a socio-economic view. Headache. 2018;58(Suppl. 1):17–32. - PubMed
-
- Alabwah Y., Ji Y., Seminowicz D.A., Quiton R.L., Masri R. Alcohol-triggered signs of migraine: an animal model. Somatosens. Mot. Res. 2016;33:35–41. - PubMed
-
- Bhatt D.K., Gupta S., Ploug K.B., Jansen-Olesen I., Olesen J. mRNA distribution of CGRP and its receptor components in the trigeminovascular system and other pain related structures in rat brain, and effect of intracerebroventricular administration of CGRP on Fos expression in the TNC. Neurosci. Lett. 2014;559:99–104. - PubMed
-
- Bolay H., Berman N.E., Akcali D. Sex-related differences in animal models of migraine headache. Headache. 2011;51:891–904. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

