Cyclic nucleotide signaling in sensory neuron hyperexcitability and chronic pain after nerve injury
- PMID: 31223142
- PMCID: PMC6565612
- DOI: 10.1016/j.ynpai.2019.100028
Cyclic nucleotide signaling in sensory neuron hyperexcitability and chronic pain after nerve injury
Abstract
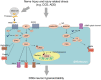
The cyclic nucleotide signaling, including cAMP-PKA and cGMP-PKG pathways, has been well known to play critical roles in regulating cellular growth, metabolism and many other intracellular processes. In recent years, more and more studies have uncovered the roles of cAMP and cGMP in the nervous system. The cAMP and cGMP signaling mediates chronic pain induced by different forms of injury and stress. Here we summarize the roles of cAMP-PKA and cGMP-PKG signaling pathways in the pathogenesis of chronic pain after nerve injury. In addition, acute dissociation and chronic compression of the dorsal root ganglion (DRG) neurons, respectively, leads to neural hyperexcitability possibly through PAR2 activation-dependent activation of cAMP-PKA pathway. Clinically, radiotherapy can effectively alleviate bone cancer pain at least partly through inhibiting the cancer cell-induced activation of cAMP-PKA pathway. Roles of cyclic nucleotide signaling in neuropathic and inflammatory pain are also seen in many other animal models and are involved in many pro-nociceptive mechanisms including the activation of hyperpolarization-activated cyclic nucleotide (HCN)-modulated ion channels and the exchange proteins directly activated by cAMP (EPAC). Further understanding the roles of cAMP and cGMP signaling in the pathogenesis of chronic pain is theoretically significant and clinically valuable for treatment of chronic pain.
Figures
Similar articles
-
Dissociation of dorsal root ganglion neurons induces hyperexcitability that is maintained by increased responsiveness to cAMP and cGMP.J Neurophysiol. 2007 Jan;97(1):15-25. doi: 10.1152/jn.00559.2006. Epub 2006 Oct 4. J Neurophysiol. 2007. PMID: 17021029
-
Chronic compression or acute dissociation of dorsal root ganglion induces cAMP-dependent neuronal hyperexcitability through activation of PAR2.Pain. 2012 Jul;153(7):1426-1437. doi: 10.1016/j.pain.2012.03.025. Epub 2012 Apr 27. Pain. 2012. PMID: 22541444
-
cAMP and cGMP contribute to sensory neuron hyperexcitability and hyperalgesia in rats with dorsal root ganglia compression.J Neurophysiol. 2006 Jan;95(1):479-92. doi: 10.1152/jn.00503.2005. Epub 2005 Aug 24. J Neurophysiol. 2006. PMID: 16120663
-
The structure of the apo cAMP-binding domain of HCN4 - a stepping stone toward understanding the cAMP-dependent modulation of the hyperpolarization-activated cyclic-nucleotide-gated ion channels.FEBS J. 2018 Jun;285(12):2182-2192. doi: 10.1111/febs.14408. Epub 2018 Mar 14. FEBS J. 2018. PMID: 29444387 Review.
-
cAMP-Dependent Protein Kinase and cGMP-Dependent Protein Kinase as Cyclic Nucleotide Effectors.Handb Exp Pharmacol. 2017;238:105-122. doi: 10.1007/164_2015_36. Handb Exp Pharmacol. 2017. PMID: 27885524 Review.
Cited by
-
Purinergic Signaling in Endometriosis-Associated Pain.Int J Mol Sci. 2020 Nov 12;21(22):8512. doi: 10.3390/ijms21228512. Int J Mol Sci. 2020. PMID: 33198179 Free PMC article. Review.
-
Action potential-independent spontaneous microdomain Ca2+ transients-mediated continuous neurotransmission regulates hyperalgesia.Proc Natl Acad Sci U S A. 2025 Jan 21;122(3):e2406741122. doi: 10.1073/pnas.2406741122. Epub 2025 Jan 17. Proc Natl Acad Sci U S A. 2025. PMID: 39823298 Free PMC article.
-
Selective targeting of peripheral cannabinoid receptors prevents behavioral symptoms and sensitization of trigeminal neurons in mouse models of migraine and medication overuse headache.Pain. 2021 Aug 1;162(8):2246-2262. doi: 10.1097/j.pain.0000000000002214. Pain. 2021. PMID: 33534356 Free PMC article.
-
Glial KCNQ K+ channels control neuronal output by regulating GABA release from glia in C. elegans.Neuron. 2024 Jun 5;112(11):1832-1847.e7. doi: 10.1016/j.neuron.2024.02.013. Epub 2024 Mar 8. Neuron. 2024. PMID: 38460523 Free PMC article.
-
ATAS Acupuncture Reduces Chemotherapy Induced Fatigue in Breast Cancer Through Regulating ADROA1 Expression: A Randomized Sham-Controlled Pilot Trial.Onco Targets Ther. 2020 Nov 17;13:11743-11754. doi: 10.2147/OTT.S272747. eCollection 2020. Onco Targets Ther. 2020. PMID: 33244238 Free PMC article.
References
-
- Abdulla F.A., Smith P.A. Axotomy- and autotomy-induced changes in Ca2+ and K+ channel currents of rat dorsal root ganglion neurons. J. Neurophysiol. 2001;85(2):644–658. - PubMed
-
- Abdulla F.A., Smith P.A. Axotomy- and autotomy-induced changes in the excitability of rat dorsal root ganglion neurons. J. Neurophysiol. 2001;85(2):630–643. - PubMed
-
- Abel T., Nguyen P.V., Barad M. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88(5):615–626. - PubMed
-
- Akins P.T., McCleskey E.W. Characterization of potassium currents in adult rat sensory neurons and modulation by opioids and cyclic AMP. Neuroscience. 1993;56(3):759–769. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

