Optimizing laboratory defined macroprolactin algorithm
- PMID: 31223260
- PMCID: PMC6559613
- DOI: 10.11613/BM.2019.020706
Optimizing laboratory defined macroprolactin algorithm
Abstract
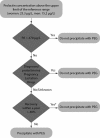
Introduction: Macroprolactinaemia is a well-known analytical problem in diagnostics of hyperprolactinaemia usually detected with polyethylene glycol (PEG) precipitation method. Since there is no harmonization in macroprolactin detection and reporting results, this study proposes and evaluates the usefulness of in-house developed algorithm. The aims were to determine the most suitable way of reporting results after PEG treatment and the possibilities of rationalizing the precipitation procedure.
Materials and methods: This is a retrospective study based on extracted data for 1136 patients. Prolactin concentrations were measured before and after PEG precipitation on Roche cobas e601. Macroprolactinaemia was defined by percentage recovery and post-PEG prolactin concentrations.
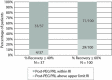
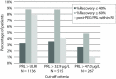
Results: Prevalence of macroprolactinaemia using recovery criteria of ≤ 40%, ≤ 60%, and post-PEG prolactin concentrations was 3.3%, 8.8% and 7.8%, respectively. Raising the cut-off value from the upper limit of the manufacturer's reference interval to 32.9 µg/L does not drastically change detected macroprolactinaemia with recovery criteria. Post-PEG prolactin concentrations showed more than half of the patients with macroprolactinaemia would be overlooked. Regardless of the criteria, a cut-off of 47.0 µg/L would miss most of the macroprolactinaemic patients. Repeated recovery measurements of follow-up patients showed there is a significant difference with mean absolute bias of 9%.
Conclusions: Post-PEG prolactin concentration with corresponding reference interval is the most suitable way of reporting results. All samples with prolactin concentration above the upper limit of the manufacturer's reference interval should be submitted to PEG precipitation. Follow-up period could be prolonged since the difference between the recoveries of repeated measurements is not clinically significant.
Keywords: hyperprolactinaemia; macroprolactin; polyethylene glycol; prolactin.
Conflict of interest statement
Potential conflict of interest: None declared.
Figures
Similar articles
-
Macroprolactin screening in 464 patients with hyperprolactinaemia.Malays J Pathol. 2022 Aug;44(2):261-267. Malays J Pathol. 2022. PMID: 36043589
-
Cross-reactivity in assays for prolactin and optimum screening policy for macroprolactinaemia.Clin Chem Lab Med. 2022 Jun 17;60(9):1365-1372. doi: 10.1515/cclm-2022-0459. Print 2022 Aug 26. Clin Chem Lab Med. 2022. PMID: 35708266
-
Reference ranges for serum total and monomeric prolactin for the current generation Abbott Architect assay.Ann Clin Biochem. 2015 Jan;52(Pt 1):61-6. doi: 10.1177/0004563214547779. Epub 2014 Jul 29. Ann Clin Biochem. 2015. PMID: 25074991
-
Determination of prolactin: the macroprolactin problem.Best Pract Res Clin Endocrinol Metab. 2013 Oct;27(5):725-42. doi: 10.1016/j.beem.2013.07.002. Epub 2013 Aug 14. Best Pract Res Clin Endocrinol Metab. 2013. PMID: 24094642 Review.
-
Prolactin Biology and Laboratory Measurement: An Update on Physiology and Current Analytical Issues.Clin Biochem Rev. 2018 Feb;39(1):3-16. Clin Biochem Rev. 2018. PMID: 30072818 Free PMC article. Review.
Cited by
-
Macroprolactinemia: a mini-review and update on clinical practice.F S Rep. 2023 May 24;4(3):245-250. doi: 10.1016/j.xfre.2023.05.005. eCollection 2023 Sep. F S Rep. 2023. PMID: 37719092 Free PMC article.

