Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life
- PMID: 31223283
- PMCID: PMC6567811
- DOI: 10.7150/ijbs.33044
Epigenetic Down-Regulation of Sirt 1 via DNA Methylation and Oxidative Stress Signaling Contributes to the Gestational Diabetes Mellitus-Induced Fetal Programming of Heart Ischemia-Sensitive Phenotype in Late Life
Abstract
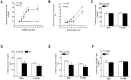
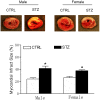
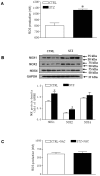
Rationale: The incidence of gestational diabetes mellitus (GDM) is increasing worldwide. However, whether and how GDM exposure induces fetal programming of adult cardiac dysfunctional phenotype, especially the underlying epigenetic molecular mechanisms and theranostics remain unclear. To address this problem, we developed a late GDM rat model. Methods: Pregnant rats were made diabetic on day 12 of gestation by streptozotocin (STZ). Experiments were conducted in 6 weeks old offspring. Results: There were significant increases in ischemia-induced cardiac infarction and gender-dependent left ventricular (LV) dysfunction in male offspring in GDM group as compared to controls. Exposure to GDM enhanced ROS level and caused a global DNA methylation in offspring cardiomyocytes. GDM attenuated cardiac Sirt 1 protein and p-Akt/Akt levels, but enhanced autophagy-related proteins expression (Atg 5 and LC3 II/LC3 I) as compared to controls. Ex-vivo treatment of DNA methylation inhibitor, 5-Aza directly inhibited Dnmt3A and enhanced Sirt 1 protein expression in fetal hearts. Furthermore, treatment with antioxidant, N-acetyl-cysteine (NAC) in offspring reversed GDM-mediated DNA hypermethylation, Sirt1 repression and autophagy-related gene protein overexpression in the hearts, and rescued GDM-induced deterioration in heart ischemic injury and LV dysfunction. Conclusion: Our data indicated that exposure to GDM induced offspring cardiac oxidative stress and DNA hypermethylation, resulting in an epigenetic down-regulation of Sirt1 gene and aberrant development of heart ischemia-sensitive phenotype, which suggests that Sirt 1-mediated signaling is the potential therapeutic target for the heart ischemic disease in offspring.
Keywords: DNA methylation; GDM; ROS; Sirt 1; heart ischemia-sensitive phenotype.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures





Similar articles
-
Reprogramming of miR-181a/DNA methylation patterns contribute to the maternal nicotine exposure-induced fetal programming of cardiac ischemia-sensitive phenotype in postnatal life.Theranostics. 2020 Sep 26;10(25):11820-11836. doi: 10.7150/thno.48297. eCollection 2020. Theranostics. 2020. PMID: 33052248 Free PMC article.
-
In utero chronic intermittent nicotine aerosol exposure increases ischemic heart injury in adult offspring via programming of Angiotensin II receptor-derived TGFβ/ROS/Akt signaling pathway.Reprod Toxicol. 2024 Sep;128:108650. doi: 10.1016/j.reprotox.2024.108650. Epub 2024 Jun 28. Reprod Toxicol. 2024. PMID: 38945500
-
STZ-induced gestational diabetes exposure alters PTEN/AKT/mTOR-mediated autophagy signaling pathway leading to increase the risk of neonatal hypoxic-ischemic encephalopathy.Reprod Toxicol. 2024 Jan;123:108494. doi: 10.1016/j.reprotox.2023.108494. Epub 2023 Oct 28. Reprod Toxicol. 2024. PMID: 38706688 Free PMC article.
-
Adenosine kinase and cardiovascular fetal programming in gestational diabetes mellitus.Biochim Biophys Acta Mol Basis Dis. 2020 Feb 1;1866(2):165397. doi: 10.1016/j.bbadis.2019.01.023. Epub 2019 Jan 27. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 30699363 Review.
-
Epigenetic programming of obesity and diabetes by in utero exposure to gestational diabetes mellitus.Nutr Rev. 2013 Oct;71 Suppl 1:S88-94. doi: 10.1111/nure.12057. Nutr Rev. 2013. PMID: 24147929 Review.
Cited by
-
Intrauterine Programming of Cardiovascular Diseases in Maternal Diabetes.Front Physiol. 2021 Nov 3;12:760251. doi: 10.3389/fphys.2021.760251. eCollection 2021. Front Physiol. 2021. PMID: 34803741 Free PMC article. Review.
-
Oxidative Stress Signaling Mediated Pathogenesis of Diabetic Cardiomyopathy.Oxid Med Cell Longev. 2022 Jan 22;2022:5913374. doi: 10.1155/2022/5913374. eCollection 2022. Oxid Med Cell Longev. 2022. PMID: 35103095 Free PMC article. Review.
-
Targeting the Epigenetic Marks in Type 2 Diabetes Mellitus: Will Epigenetic Therapy Be a Valuable Adjunct to Pharmacotherapy?Diabetes Metab Syndr Obes. 2024 Sep 21;17:3557-3576. doi: 10.2147/DMSO.S479077. eCollection 2024. Diabetes Metab Syndr Obes. 2024. PMID: 39323929 Free PMC article. Review.
-
Advances in the role of silence information regulator family in pathological pregnancy.Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021 Jun 25;50(3):335-344. doi: 10.3724/zdxbyxb-2021-0183. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2021. PMID: 34402258 Free PMC article. Review. English.
-
Fetal e-cigarette exposure programs a neonatal brain hypoxic-ischemic sensitive phenotype via altering DNA methylation patterns and autophagy signaling pathway.Am J Physiol Regul Integr Comp Physiol. 2021 Nov 1;321(5):R791-R801. doi: 10.1152/ajpregu.00207.2021. Epub 2021 Sep 15. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34524928 Free PMC article.
References
-
- Russo LM, Nobles C, Ertel KA, Chasan-Taber L, Whitcomb BW. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol. 2015;125:576–82. - PubMed
-
- Dervisoglu P, Kosecik M, Kumbasar S. Effects of gestational and pregestational diabetes mellitus on the foetal heart: a cross-sectional study. J Obstet Gynaecol. 2018;38:408–12. - PubMed
-
- Leybovitz-Haleluya N, Wainstock T, Landau D, Sheiner E. Maternal gestational diabetes mellitus and the risk of subsequent pediatric cardiovascular diseases of the offspring: a population-based cohort study with up to 18 years of follow up. Acta Diabetol; 2018. - PubMed
-
- Robertson KD. DNA methylation and human disease. Nat Rev Genet. 2005;6:597–610. - PubMed

