Anti-Inflammatory Effect of an Apigenin-Maillard Reaction Product in Macrophages and Macrophage-Endothelial Cocultures
- PMID: 31223429
- PMCID: PMC6541947
- DOI: 10.1155/2019/9026456
Anti-Inflammatory Effect of an Apigenin-Maillard Reaction Product in Macrophages and Macrophage-Endothelial Cocultures
Abstract
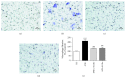
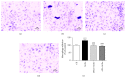
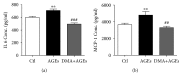
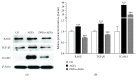
Chronic inflammation is involved in the progression of various diseases, while dietary flavonoids are reported to possess antioxidative and anti-inflammatory properties against age-related diseases. Previously, an apigenin-Maillard reaction product, dimethylglyoxal apigenin (DMA), was identified by us and demonstrated to be antioxidative. In this study, we investigated the inhibitory effect of DMA on advanced glycation end product- (AGE-) induced inflammation in macrophages and macrophage-endothelial cocultures. Results showed that DMA remarkably inhibited the mRNA and protein expression of receptor for AGEs (RAGE), thereby inhibiting the production of ROS and proinflammatory cytokines, including tumor necrosis factor- (TNF-) α, interleukin (IL) 1, IL 6, and monocyte chemoattractant protein- (MCP-) 1 in RAW 264.7 cells. In the coculture system which was performed in the Boyden chamber, macrophage infiltration and adhesion to endothelial cells were significantly suppressed by DMA. Further study indicated that DMA decreased AGE-evoked IL 6 and MCP-1 secretion, which might be achieved through RAGE and its downstream-regulated transforming growth factor- (TGF-) β1 and intercellular adhesion molecule (ICAM) 1 expression in the coculture system. In conclusion, our study demonstrates that DMA, a thermally induced compound, has anti-inflammatory activity in both macrophages and macrophage-endothelial cocultures, offering a promising approach for slowing down the development of chronic diseases.
Figures




Similar articles
-
The anti-inflammation effect of Moutan Cortex on advanced glycation end products-induced rat mesangial cells dysfunction and High-glucose-fat diet and streptozotocin-induced diabetic nephropathy rats.J Ethnopharmacol. 2014;151(1):591-600. doi: 10.1016/j.jep.2013.11.015. Epub 2013 Nov 21. J Ethnopharmacol. 2014. PMID: 24269777
-
Anti-inflammatory mechanisms of apigenin: inhibition of cyclooxygenase-2 expression, adhesion of monocytes to human umbilical vein endothelial cells, and expression of cellular adhesion molecules.Arch Pharm Res. 2007 Oct;30(10):1318-27. doi: 10.1007/BF02980273. Arch Pharm Res. 2007. PMID: 18038911
-
Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages.Biomed Pharmacother. 2017 Sep;93:1205-1212. doi: 10.1016/j.biopha.2017.07.054. Epub 2017 Jul 20. Biomed Pharmacother. 2017. PMID: 28738536
-
Postprandial lipoproteins and the molecular regulation of vascular homeostasis.Prog Lipid Res. 2013 Oct;52(4):446-64. doi: 10.1016/j.plipres.2013.06.001. Epub 2013 Jun 15. Prog Lipid Res. 2013. PMID: 23774609 Review.
-
Probing into Therapeutic Anti-cancer Potential of Apigenin: Recent Trends and Future Directions.Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):124-133. doi: 10.2174/1872213X13666190816160240. Recent Pat Inflamm Allergy Drug Discov. 2019. PMID: 31418666 Review.
Cited by
-
Dicarbonyl Stress at the Crossroads of Healthy and Unhealthy Aging.Cells. 2019 Jul 19;8(7):749. doi: 10.3390/cells8070749. Cells. 2019. PMID: 31331077 Free PMC article. Review.
-
Anti-inflammatory activities of flavonoid derivates.ADMET DMPK. 2023 Jul 26;11(3):331-359. doi: 10.5599/admet.1918. eCollection 2023. ADMET DMPK. 2023. PMID: 37829324 Free PMC article. Review.
-
Necroptosis in Pneumonia: Therapeutic Strategies and Future Perspectives.Viruses. 2024 Jan 7;16(1):94. doi: 10.3390/v16010094. Viruses. 2024. PMID: 38257794 Free PMC article. Review.
-
Apigenin attenuates atherosclerosis and non-alcoholic fatty liver disease through inhibition of NLRP3 inflammasome in mice.Sci Rep. 2023 May 17;13(1):7996. doi: 10.1038/s41598-023-34654-2. Sci Rep. 2023. PMID: 37198205 Free PMC article.
-
Facilitating Reparative Dentin Formation Using Apigenin Local Delivery in the Exposed Pulp Cavity.Front Physiol. 2021 Dec 10;12:773878. doi: 10.3389/fphys.2021.773878. eCollection 2021. Front Physiol. 2021. PMID: 34955887 Free PMC article.
References
-
- Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. International Journal of Molecular Sciences. 2007;8(9):950–988. doi: 10.3390/i8090950. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

