Biofabrication strategies for 3D in vitro models and regenerative medicine
- PMID: 31223488
- PMCID: PMC6586020
- DOI: 10.1038/s41578-018-0006-y
Biofabrication strategies for 3D in vitro models and regenerative medicine
Abstract
Organs are complex systems composed of different cells, proteins and signalling molecules that are arranged in a highly ordered structure to orchestrate a myriad of functions in our body. Biofabrication strategies can be applied to engineer 3D tissue models in vitro by mimicking the structure and function of native tissue through the precise deposition and assembly of materials and cells. This approach allows the spatiotemporal control over cell-cell and cell-extracellular matrix communication and thus the recreation of tissue-like structures. In this Review, we examine biofabrication strategies for the construction of functional tissue replacements and organ models, focusing on the development of biomaterials, such as supramolecular and photosensitive materials, that can be processed using biofabrication techniques. We highlight bioprinted and bioassembled tissue models and survey biofabrication techniques for their potential to recreate complex tissue properties, such as shape, vasculature and specific functionalities. Finally, we discuss challenges, such as scalability and the foreign body response, and opportunities in the field and provide an outlook to the future of biofabrication in regenerative medicine.
Conflict of interest statement
Competing interests The authors declare no competing interests.
Figures
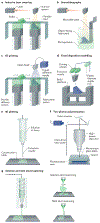






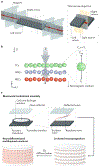
References
-
- Gomes ME, Rodrigues MT, Domingues RMA & Reis RL Tissue engineering and regenerative medicine: new trends and directions-a year in review. Tissue Eng. Part B Rev 23, 211–224 (2017). - PubMed
-
- Tschugg A et al. A prospective randomized multicenter phase I/II clinical trial to evaluate safety and efficacy of NOVOCART disk plus autologous disk chondrocyte transplantation in the treatment of nucleotomized and degenerative lumbar disks to avoid secondary disease: safety results of Phase I-a short report. Neurosurg. Rev 40, 155–162 - PMC - PubMed
-
- Martin I et al. The survey on cellular and engineered tissue therapies in Europe in 2013. Tissue Eng. A 22, 5–16 (2016). - PubMed
-
- Groll J et al. Biofabrication: reappraising the definition of an evolving field. Biofabrication 8, 013001 (2016). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
