Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase
- PMID: 31225454
- PMCID: PMC6551803
- DOI: 10.15698/cst2018.11.162
Making the final cut: pathogenic amyloid-β peptide generation by γ-secretase
Abstract
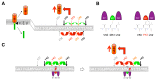
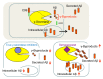
Alzheimer´s disease (AD) is a devastating neurodegenerative disease of the elderly population. Genetic evidence strongly suggests that aberrant generation and/or clearance of the neurotoxic amyloid-β peptide (Aβ) is triggering the disease. Aβ is generated from the amyloid precursor protein (APP) by the sequential cleavages of β- and γ-secretase. The latter cleavage by γ-secretase, a unique and fascinating four-component protease complex, occurs in the APP transmembrane domain thereby releasing Aβ species of 37-43 amino acids in length including the longer, highly pathogenic peptides Aβ42 and Aβ43. The lack of a precise understanding of Aβ generation as well as of the functions of other γ-secretase substrates has been one factor underlying the disappointing failure of γ-secretase inhibitors in clinical trials, but on the other side also been a major driving force for structural and in depth mechanistic studies on this key AD drug target in the past few years. Here we review recent breakthroughs in our understanding of how the γ-secretase complex recognizes substrates, of how it binds and processes β-secretase cleaved APP into different Aβ species, as well as the progress made on a question of outstanding interest, namely how clinical AD mutations in the catalytic subunit presenilin and the γ-secretase cleavage region of APP lead to relative increases of Aβ42/43. Finally, we discuss how the knowledge emerging from these studies could be used to therapeutically target this enzyme in a safe way.
Keywords: Alzheimer's disease; amyloid β-peptide; presenilin; γ-secretase.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
