Withaferin A inhibits adipogenesis in 3T3-F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice
- PMID: 31226166
- PMCID: PMC6588247
- DOI: 10.1371/journal.pone.0218792
Withaferin A inhibits adipogenesis in 3T3-F442A cell line, improves insulin sensitivity and promotes weight loss in high fat diet-induced obese mice
Abstract
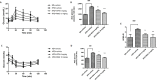
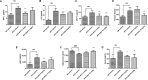
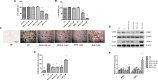
The increased prevalence of obesity and associated insulin resistance calls for effective therapeutic treatment of metabolic diseases. The current PPARγ-targeting antidiabetic drugs have undesirable side effects. The present study investigated the anti-diabetic and anti-obesity effects of withaferin A (WFA) in diet-induced obese (DIO) C57BL/6J mice and also the anti-adipogenic effect of WFA in differentiating 3T3- F442A cells. DIO mice were treated with WFA (6 mg/kg) or rosiglitazone (10 mg/kg) for 8 weeks. At the end of the treatment period, metabolic profile, liver function and inflammatory parameters were obtained. Expression of selective genes controlling insulin signaling, inflammation, adipogenesis, energy expenditure and PPARγ phosphorylation-regulated genes in epididymal fats were analyzed. Furthermore, the anti-adipogenic effect of WFA was evaluated in 3T3- F442A cell line. WFA treatment prevented weight gain without affecting food or caloric intake in DIO mice. WFA-treated group also exhibited lower epididymal and mesenteric fat pad mass, an improvement in lipid profile and hepatic steatosis and a reduction in serum inflammatory cytokines. Insulin resistance was reduced as shown by an improvement in glucose and insulin tolerance and serum adiponectin. WFA treatment upregulated selective insulin signaling (insr, irs1, slc2a4 and pi3k) and PPARγ phosphorylation-regulated (car3, selenbp1, aplp2, txnip, and adipoq) genes, downregulated inflammatory (tnf-α and il-6) genes and altered energy expenditure controlling (tph2 and adrb3) genes. In 3T3- F442A cell line, withaferin A inhibited adipogenesis as indicated by a decrease in lipid accumulation in differentiating adipocytes and protein expression of PPARγ and C/EBPα. The effect of rosiglitazone on physiological and lipid profiles, insulin resistance, some genes expression and differentiating adipocytes were markedly different. Our data suggest that WFA is a promising therapeutic agent for both diabetes and obesity.
Conflict of interest statement
The authors have declared that no competing interest exists.
Figures


References
-
- Miller MR, Pereira RI, Langefeld CD, Lorenzo C, Rotter JI, Chen Y-DI, et al. Levels of Free Fatty Acids (FFA) Are Associated with Insulin Resistance But Do Not Explain the Relationship between Adiposity and Insulin Resistance in Hispanic Americans: The IRAS Family Study. The Journal of Clinical Endocrinology and Metabolism. 2012;97(9):3285–91. 10.1210/jc.2012-1318 PMC3431582. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

