Poly(furfuryl alcohol)-Polycaprolactone Blends
- PMID: 31226802
- PMCID: PMC6630956
- DOI: 10.3390/polym11061069
Poly(furfuryl alcohol)-Polycaprolactone Blends
Abstract
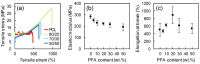
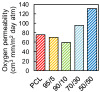
Poly(furfuryl alcohol) (PFA) is a bioresin synthesized from furfuryl alcohol (FA) that is derived from renewable saccharide-rich biomass. In this study, we compounded this bioresin with polycaprolactone (PCL) for the first time, introducing new functional polymer blends. Although PCL is biodegradable, its production relies on petroleum precursors such as cyclohexanone oils. With the method proposed herein, this dependence on petroleum-derived precursors/monomers is reduced by using PFA without significantly modifying some important properties of the PCL. Polymer blend films were produced by simple solvent casting. The blends were characterized in terms of surface topography by atomic force microscopy (AFM), chemical interactions between PCL and PFA by attenuated total reflection-Fourier transform infrared (ATR-FTIR), crystallinity by XRD, thermal properties by differential scanning calorimetry (DSC), and mechanical properties by tensile tests and biocompatibility by direct and indirect toxicity tests. PFA was found to improve the gas barrier properties of PCL without compromising its mechanical properties, and it demonstrated sustained antioxidant effect with excellent biocompatibility. Our results indicate that these new blends can be potentially used in diverse applications ranging from food packing to biomedical devices.
Keywords: antioxidant polymer; biocompatibility; food packaging; poly(furfuryl alcohol); polycaprolactone.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Mariscal R., Maireles-Torres P., Ojeda M., Sádaba I., López Granados M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016;9:1144–1189. doi: 10.1039/C5EE02666K. - DOI
-
- Chan X., Yang P., Ooi C., Cen J., Orlov A., Kim T. Separation and purification of furfuryl alcohol monomer and oligomers using a two-phase extracting process. ACS Sustain. Chem. Eng. 2016;4:4084–4088. doi: 10.1021/acssuschemeng.6b01067. - DOI
-
- Gardikes J.J., Young D.K. Composition Containing Furfuryl Alcohol and Use Thereof in Foundry Binders. 4,371,648. U.S. Patent. 1983 Feb 1;
LinkOut - more resources
Full Text Sources
Miscellaneous

