Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance
- PMID: 31226830
- PMCID: PMC6630611
- DOI: 10.3390/nano9060900
Heterostructured NiO/ZnO Nanorod Arrays with Significantly Enhanced H2S Sensing Performance
Abstract
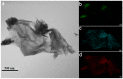
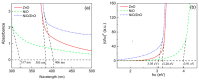
H2S gas sensors were fabricated using p-n heterojunctions of NiO/ZnO, in which the ZnO nanorod arrays were wrapped with NiO nanosheets via a hydrothermal synthesis method. When the H2S gas molecules were adsorbed and then oxidized on the ZnO surfaces, the free electrons were released. The increase in the electron concentration on the ZnO boosts the transport speed of the electrons on both sides of the NiO/ZnO p-n junction, which significantly improved the sensing performance and selectivity for H2S detection, if compared with sensors using the pure ZnO nanorod arrays. The response to 20 ppm of H2S was 21.3 at 160 °C for the heterostructured NiO/ZnO sensor, and the limit of detection was 0.1 ppm. We found that when the sensor was exposed to H2S at an operating temperature below 160 °C, the resistance of the sensor significantly decreased, indicating its n-type semiconductor nature, whereas when the operating temperature was above 160 °C, the resistance significantly increased, indicating its p-type semiconductor nature. The sensing mechanism of the NiO/ZnO heterostructured H2S gas sensor was discussed in detail.
Keywords: H2S gas sensor; NiO; ZnO; nanorods; p-n junction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures









References
-
- Xie F.Y., Cao X.Q., Qu F.L., Asiri A.M., Sun X.P. Cobalt nitride nanowire array as an efficient electrochemical sensor for glucose and H2O2 detection. Sens. Actuators B Chem. 2018;255:1254–1261. doi: 10.1016/j.snb.2017.08.098. - DOI
-
- Li Z.J., Niu X.Y., Lin Z.J., Wang N.N., Shen H.H., Liu W., Sun K., Fu Y.Q., Wang Z.G. Hydrothermally synthesized CeO2 nanowires for H2S sensing at room temperature. J. Alloys Compd. 2016;682:647–653. doi: 10.1016/j.jallcom.2016.04.311. - DOI
-
- Kim J., Yong K. Mechanism Study of ZnO Nanorod-Bundle Sensors for H2S Gas Sensing. J. Phys. Chem. C. 2011;115:7218–7224. doi: 10.1021/jp110129f. - DOI
-
- Tian H.L., Fan H.Q., Li M.M., Ma L.T. Zeolitic Imidazolate Framework Coated ZnO Nanorods as Molecular Sieving to Improve Selectivity of Formaldehyde Gas Sensor. ACS Sens. 2016;1:243–250. doi: 10.1021/acssensors.5b00236. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

