Removal of Phenolic Compounds from Water Using Copper Ferrite Nanosphere Composites as Fenton Catalysts
- PMID: 31226850
- PMCID: PMC6631195
- DOI: 10.3390/nano9060901
Removal of Phenolic Compounds from Water Using Copper Ferrite Nanosphere Composites as Fenton Catalysts
Abstract
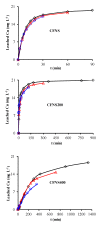
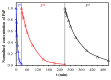
Copper ferrites containing Cu+ ions can be highly active heterogeneous Fenton catalysts due to synergic effects between Fe and Cu ions. Therefore, a method of copper ferrite nanosphere (CFNS) synthesis was selected that also permits the formation of cuprite, obtaining a CFNS composite that was subsequently calcined up to 400 °C. Composites were tested as Fenton catalysts in the mineralization of phenol (PHE), p-nitrophenol (PNP) and p-aminophenol (PAP). Catalysts were characterized by transmission electron microscopy (TEM), scanning electron microscopy (SEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), X-ray photoelectron spectroscopy (XPS) and magnetic measurements. Degradation of all phenols was practically complete at 95% total organic carbon (TOC) removal. Catalytic activity increased in the order PHE < PNP < PAP and decreased when the calcination temperature was raised; this order depended on the electronic effects of the substituents of phenols. The as-prepared CFNS showed the highest catalytic activity due to the presence of cubic copper ferrite and cuprite. The Cu+ surface concentration decreased after calcination at 200 °C, diminishing the catalytic activity. Cuprite alone showed a lower activity than the CFNS composite and the homogeneous Fenton reaction had almost no influence on its overall activity. CFNS activity decreased with its reutilization due to the disappearance of the cuprite phase. Degradation pathways are proposed for the phenols.
Keywords: Fenton reaction; copper ferrite; cuprite; phenols; synergic effects.
Conflict of interest statement
The authors declare no conflict of interest.
Figures










References
-
- Muñoz M., de Pedro Z.M., Casas J.A., Rodríguez J.J. Preparation of magnetite-based catalysts and their application in heterogeneous Fenton oxidation—A review. Appl. Catal. B Environ. 2015;176–177:249–265. doi: 10.1016/j.apcatb.2015.04.003. - DOI
-
- Pignatello J.J., Oliveros E., MacKay A. Advanced oxidation processes for organic contaminant destruction based on the Fenton reaction and related chemistry. Crit. Rev. Environ. Sci. Technol. 2016;36:1–84. doi: 10.1080/10643380500326564. - DOI
-
- Sun Y., Yang Z., Tian P., Sheng Y., Xu J., Han Y.F. Oxidative degradation of nitrobenzene by a Fenton-like reaction with Fe-Cu bimetallic catalysts. Appl. Catal. B Environ. 2019;244:1–10. doi: 10.1016/j.apcatb.2018.11.009. - DOI
-
- Nichela D.A., Berkovic A.M., Costante M.R., Juliarena M.P., García Einschlag F.S. Nitrobenzene degradation in Fenton-like systems using Cu(II) as catalyst. Comparison between Cu(II)-and Fe(III)-based systems. Chem. Eng. J. 2013;228:1148–1157. doi: 10.1016/j.cej.2013.05.002. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

