Evaluating the safety, effectiveness and acceptability of treatment of incomplete second-trimester abortion using misoprostol provided by midwives compared with physicians: study protocol for a randomized controlled equivalence trial
- PMID: 31227019
- PMCID: PMC6588936
- DOI: 10.1186/s13063-019-3490-5
Evaluating the safety, effectiveness and acceptability of treatment of incomplete second-trimester abortion using misoprostol provided by midwives compared with physicians: study protocol for a randomized controlled equivalence trial
Abstract
Background: A large proportion of abortion-related mortality and morbidity occurs in the second trimester of pregnancy. The Uganda Ministry of Health policy restricts management of second-trimester incomplete abortion to physicians who are few and unequally distributed, with most practicing in urban regions. Unsafe and outdated methods like sharp curettage are frequently used. Medical management of second-trimester post-abortion care by midwives offers an advantage given the difficulty in providing surgical management in low-income settings and current health worker shortages. The study aims to assess the safety, effectiveness and acceptability of treatment of incomplete second-trimester abortion using misoprostol provided by midwives compared with physicians.
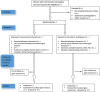
Methods: A randomized controlled equivalence trial implemented at eight hospitals and health centers in Central Uganda will include 1192 eligible women with incomplete abortion of uterine size > 12 weeks up to 18 weeks. Each participant will be randomly assigned to undergo a clinical assessment and treatment by either a midwife (intervention arm) or a physician (control arm). Enrolled participants will receive 400 μg misoprostol administered sublingually every 3 h up to five doses within 24 h at the health facility until a complete abortion is confirmed. Women who do not achieve complete abortion within 24 h will undergo surgical uterine evacuation. Pre discharge, participants will receive contraceptive counseling and information on what to expect in terms of side effects and signs of complications, with follow-up 14 days later to assess secondary outcomes. Analyses will be by intention to treat. Background characteristics and outcomes will be presented using descriptive statistics. Differences between groups will be analyzed using risk difference (95% confidence interval) and equivalence established if this lies between the predefined range of - 5% and + 5%. Chi-square tests will be used for comparison of outcome and t tests used to compare mean values. P ≤ 0.05 will be considered statistically significant.
Discussion: Our study will provide evidence to inform national and international policies, standard care guidelines and training program curricula on treatment of second-trimester incomplete abortion for improved access.
Trial registration: ClinicalTrials.gov, NCT03622073 . Registered on 9 August 2018.
Keywords: Contraception; Incomplete abortion; Misoprostol; Post abortion care; Second trimester; Task sharing; Uganda.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
Similar articles
-
Comparison of the effectiveness and safety of treatment of incomplete second trimester abortion with misoprostol provided by midwives and physicians: a randomised, controlled, equivalence trial in Uganda.Lancet Glob Health. 2022 Oct;10(10):e1505-e1513. doi: 10.1016/S2214-109X(22)00312-6. Epub 2022 Aug 26. Lancet Glob Health. 2022. PMID: 36030801 Free PMC article. Clinical Trial.
-
Evaluating women's acceptability of treatment of incomplete second trimester abortion using misoprostol provided by midwives compared with physicians: a mixed methods study.BMC Womens Health. 2022 Nov 5;22(1):434. doi: 10.1186/s12905-022-02027-y. BMC Womens Health. 2022. PMID: 36335344 Free PMC article. Clinical Trial.
-
Comparison of treatment of incomplete abortion with misoprostol by physicians and midwives at district level in Uganda: a randomised controlled equivalence trial.Lancet. 2015 Jun 13;385(9985):2392-8. doi: 10.1016/S0140-6736(14)61935-8. Epub 2015 Mar 27. Lancet. 2015. PMID: 25817472 Clinical Trial.
-
Medical management of induced and incomplete first-trimester abortion by non-physicians in low- and middle-income countries: A systematic review and meta-analysis of randomized controlled trials.Acta Obstet Gynecol Scand. 2021 Apr;100(4):718-726. doi: 10.1111/aogs.14134. Epub 2021 Mar 18. Acta Obstet Gynecol Scand. 2021. PMID: 33724458
-
Second-trimester postabortion care for ruptured membranes, fetal demise, and incomplete abortion.Int J Gynaecol Obstet. 2015 May;129(2):98-103. doi: 10.1016/j.ijgo.2014.11.011. Epub 2015 Jan 19. Int J Gynaecol Obstet. 2015. PMID: 25660084 Review.
Cited by
-
The efficacy and influence factors analysis of Mifepristone combined with estrogen-progesterone in the treatment of incomplete abortion.Medicine (Baltimore). 2023 Apr 7;102(14):e33532. doi: 10.1097/MD.0000000000033532. Medicine (Baltimore). 2023. PMID: 37026901 Free PMC article.
-
Comparison of the effectiveness and safety of treatment of incomplete second trimester abortion with misoprostol provided by midwives and physicians: a randomised, controlled, equivalence trial in Uganda.Lancet Glob Health. 2022 Oct;10(10):e1505-e1513. doi: 10.1016/S2214-109X(22)00312-6. Epub 2022 Aug 26. Lancet Glob Health. 2022. PMID: 36030801 Free PMC article. Clinical Trial.
-
Exploring health care providers' experiences of and perceptions towards the use of misoprostol for management of second trimester incomplete abortion in Central Uganda.PLoS One. 2022 May 19;17(5):e0268812. doi: 10.1371/journal.pone.0268812. eCollection 2022. PLoS One. 2022. PMID: 35587492 Free PMC article.
-
Evaluating women's acceptability of treatment of incomplete second trimester abortion using misoprostol provided by midwives compared with physicians: a mixed methods study.BMC Womens Health. 2022 Nov 5;22(1):434. doi: 10.1186/s12905-022-02027-y. BMC Womens Health. 2022. PMID: 36335344 Free PMC article. Clinical Trial.
-
Second trimester post-abortion family planning uptake and associated factors in 14 public health facilities in Central Uganda: a cross-sectional study.Contracept Reprod Med. 2023 Jan 14;8(1):4. doi: 10.1186/s40834-022-00199-4. Contracept Reprod Med. 2023. PMID: 36639699 Free PMC article.
References
-
- World Health Organization, World Bank, United Nations Population Fund & United Nations Children’s Fund ( UNICEF). Trends in maternal mortality: 1990-2015: estimates from WHO, UNICEF, UNFPA, World Bank Group and the United Nations Population Division. World Health Organization. 2015. http://www.who.int/iris/handle/10665/194254.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical