Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use
- PMID: 31227636
- PMCID: PMC6622431
- DOI: 10.1681/ASN.2018121254
Daratumumab in Sensitized Kidney Transplantation: Potentials and Limitations of Experimental and Clinical Use
Abstract
Background: Donor-specific antibodies are associated with increased risk of antibody-mediated rejection and decreased allograft survival. Therefore, reducing the risk of these antibodies remains a clinical need in transplantation. Plasma cells are a logical target of therapy given their critical role in antibody production.
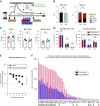
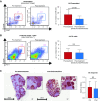
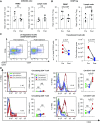
Methods: To target plasma cells, we treated sensitized rhesus macaques with daratumumab (anti-CD38 mAb). Before transplant, we sensitized eight macaques with two sequential skin grafts from MHC-mismatched donors; four of them were also desensitized with daratumumab and plerixafor (anti-CXCR4). We also treated two patients with daratumumab in the context of transplant.
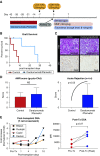
Results: The animals treated with daratumumab had significantly reduced donor-specific antibody levels compared with untreated controls (57.9% versus 13% reduction; P<0.05) and prolonged renal graft survival (28.0 days versus 5.2 days; P<0.01). However, the reduction in donor-specific antibodies was not maintained because all recipients demonstrated rapid rebound of antibodies, with profound T cell-mediated rejection. In the two clinical patients, a combined heart and kidney transplant recipient with refractory antibody-mediated rejection and a highly sensitized heart transplant candidate, we also observed a significant decrease in class 1 and 2 donor-specific antibodies that led to clinical improvement of antibody-mediated rejection and to heart graft access.
Conclusions: Targeting CD38 with daratumumab significantly reduced anti-HLA antibodies and anti-HLA donor-specific antibodies in a nonhuman primate model and in two transplant clinical cases before and after transplant. This supports investigation of daratumumab as a potential therapeutic strategy; however, further research is needed regarding its use for both antibody-mediated rejection and desensitization.
Keywords: antibody-mediated rejection; daratumumab; desensitization; nonhuman primate; plasma cell.
Copyright © 2019 by the American Society of Nephrology.
Figures



References
-
- Port FK, Wolfe RA, Mauger EA, Berling DP, Jiang K: Comparison of survival probabilities for dialysis patients vs cadaveric renal transplant recipients. JAMA 270: 1339–1343, 1993 - PubMed
-
- Russell JD, Beecroft ML, Ludwin D, Churchill DN: The quality of life in renal transplantation—a prospective study. Transplantation 54: 656–660, 1992 - PubMed
-
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, et al. .: Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med 341: 1725–1730, 1999 - PubMed
-
- Loupy A, Lefaucheur C, Vernerey D, Prugger C, Duong van Huyen JP, Mooney N, et al. .: Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 369: 1215–1226, 2013 - PubMed
-
- Bentall A, Cornell LD, Gloor JM, Park WD, Gandhi MJ, Winters JL, et al. .: Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 13: 76–85, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

