Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor
- PMID: 31227695
- PMCID: PMC6588579
- DOI: 10.1038/s41467-019-10401-y
Monitoring biomolecule concentrations in tissue using a wearable droplet microfluidic-based sensor
Abstract
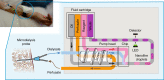
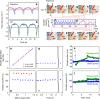
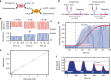
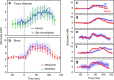
Knowing how biomarker levels vary within biological fluids over time can produce valuable insight into tissue physiology and pathology, and could inform personalised clinical treatment. We describe here a wearable sensor for monitoring biomolecule levels that combines continuous fluid sampling with in situ analysis using wet-chemical assays (with the specific assay interchangeable depending on the target biomolecule). The microfluidic device employs a droplet flow regime to maximise the temporal response of the device, using a screw-driven push-pull peristaltic micropump to robustly produce nanolitre-sized droplets. The fully integrated sensor is contained within a small (palm-sized) footprint, is fully autonomous, and features high measurement frequency (a measurement every few seconds) meaning deviations from steady-state levels are quickly detected. We demonstrate how the sensor can track perturbed glucose and lactate levels in dermal tissue with results in close agreement with standard off-line analysis and consistent with changes in peripheral blood levels.
Conflict of interest statement
X.N. is the founder of SouthWestSensor Ltd., which develops chemical sensor technology based on droplet microfluidics. Remaining authors declare no competing interests.
Figures

References
-
- Rogers, M. L. & Boutelle, M. G. In Annual Review of Analytical Chemistry, Vol 6.Annual Review of AnalyticalChemistry (eds R. G. Cooks & J. E. Pemberton) 427–453 (Annual Reviews, Palo Alto, 2013). - PubMed
-
- Schazmann B, et al. A wearable electrochemical sensor for the real-time measurement of sweat sodium concentration. Anal. Methods. 2010;2:342–348. doi: 10.1039/b9ay00184k. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

