α7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins
- PMID: 31227712
- PMCID: PMC6588605
- DOI: 10.1038/s41467-019-10723-x
α7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins
Abstract
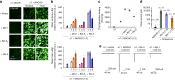
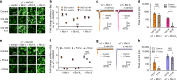
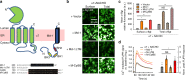
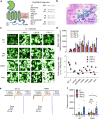
Nicotinic acetylcholine receptors (nAChRs) mediate and modulate synaptic transmission throughout the brain, and contribute to learning, memory, and behavior. Dysregulation of α7-type nAChRs in neuropsychiatric as well as immunological and oncological diseases makes them attractive targets for pharmaceutical development. Recently, we identified NACHO as an essential chaperone for α7 nAChRs. Leveraging the robust recombinant expression of α7 nAChRs with NACHO, we utilized genome-wide cDNA library screening and discovered that several anti-apoptotic Bcl-2 family proteins further upregulate receptor assembly and cell surface expression. These effects are mediated by an intracellular motif on α7 that resembles the BH3 binding domain of pro-apoptotic Bcl-2 proteins, and can be blocked by BH3 mimetic Bcl-2 inhibitors. Overexpression of Bcl-2 member Mcl-1 in neurons enhanced surface expression of endogenous α7 nAChRs, while a combination of chemotherapeutic Bcl2-inhibitors suppressed neuronal α7 receptor assembly. These results demonstrate that Bcl-2 proteins link α7 nAChR assembly to cell survival pathways.
Conflict of interest statement
The authors declare no competing interests except that authors are all full-time employees of Janssen Pharmaceutical Companies of Johnson and Johnson.
Figures
Similar articles
-
Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly.Molecules. 2022 Jul 15;27(14):4527. doi: 10.3390/molecules27144527. Molecules. 2022. PMID: 35889400 Free PMC article. Review.
-
Genetic deletion of the adenosine A(2A) receptor prevents nicotine-induced upregulation of α7, but not α4β2* nicotinic acetylcholine receptor binding in the brain.Neuropharmacology. 2013 Aug;71:228-36. doi: 10.1016/j.neuropharm.2013.03.023. Epub 2013 Apr 11. Neuropharmacology. 2013. PMID: 23583933
-
Brain α7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO.Neuron. 2016 Mar 2;89(5):948-55. doi: 10.1016/j.neuron.2016.01.018. Epub 2016 Feb 11. Neuron. 2016. PMID: 26875622
-
NACHO Mediates Nicotinic Acetylcholine Receptor Function throughout the Brain.Cell Rep. 2017 Apr 25;19(4):688-696. doi: 10.1016/j.celrep.2017.04.008. Cell Rep. 2017. PMID: 28445721
-
Advances in the discovery of novel positive allosteric modulators of the alpha7 nicotinic acetylcholine receptor.Recent Pat CNS Drug Discov. 2007 Jun;2(2):99-106. doi: 10.2174/157488907780832751. Recent Pat CNS Drug Discov. 2007. PMID: 18221220 Review.
Cited by
-
Distinct Evolutionary Trajectories of Neuronal and Hair Cell Nicotinic Acetylcholine Receptors.Mol Biol Evol. 2020 Apr 1;37(4):1070-1089. doi: 10.1093/molbev/msz290. Mol Biol Evol. 2020. PMID: 31821508 Free PMC article.
-
Imaging Cholinergic Receptors in the Brain by Positron Emission Tomography.J Med Chem. 2023 Aug 24;66(16):10889-10916. doi: 10.1021/acs.jmedchem.3c00573. Epub 2023 Aug 15. J Med Chem. 2023. PMID: 37583063 Free PMC article. Review.
-
The Hair Cell α9α10 Nicotinic Acetylcholine Receptor: Odd Cousin in an Old Family.Front Cell Neurosci. 2021 Nov 15;15:785265. doi: 10.3389/fncel.2021.785265. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34867208 Free PMC article. Review.
-
Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly.Molecules. 2022 Jul 15;27(14):4527. doi: 10.3390/molecules27144527. Molecules. 2022. PMID: 35889400 Free PMC article. Review.
-
SARS-CoV-2 Spike Protein Downregulates Cell Surface α7nAChR through a Helical Motif in the Spike Neck.ACS Chem Neurosci. 2023 Feb 15;14(4):689-698. doi: 10.1021/acschemneuro.2c00610. Epub 2023 Feb 6. ACS Chem Neurosci. 2023. PMID: 36745901 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

