Phrixotrix luciferase and 6'-aminoluciferins reveal a larger luciferin phenolate binding site and provide novel far-red combinations for bioimaging purposes
- PMID: 31227722
- PMCID: PMC6588592
- DOI: 10.1038/s41598-019-44534-3
Phrixotrix luciferase and 6'-aminoluciferins reveal a larger luciferin phenolate binding site and provide novel far-red combinations for bioimaging purposes
Abstract
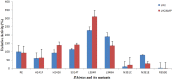
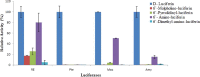
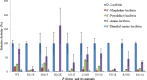
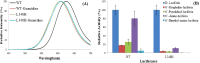
How the unique luciferase of Phrixothrix hirtus (PxRE) railroad worm catalyzes the emission of red bioluminescence using the same luciferin of fireflies, remains a mystery. Although PxRE luciferase is a very attractive tool for bioanalysis and bioimaging in hemoglobin rich tissues, it displays lower quantum yield (15%) when compared to green emitting luciferases (>40%). To identify which parts of PxRE luciferin binding site (LBS) determine bioluminescence color, and to develop brighter and more red-shifted emitting luciferases, we compared the effects of site-directed mutagenesis and of larger 6'-substituted aminoluciferin analogues (6'-morpholino- and 6'-pyrrolidinyl-LH) on the bioluminescence properties of PxRE and green-yellow emitting beetle luciferases. The effects of mutations in the benzothiazolyl and thiazolyl parts of PxRE LBS on the KM and catalytic efficiencies, indicated their importance for luciferin binding and catalysis. However, the absence of effects on the bioluminescence spectrum indicated a less interactive LBS in PxRE during light emission. Mutations at the bottom of LBS of PxRE blue-shifted the spectra and increased catalytic efficiency, suggesting that lack of interactions of this part of LBS with excited oxyluciferin phenolate underlie red light emission. The much higher bioluminescence activity and red-shifted spectra of PxRE luciferase with 6'-morpholino- (634 nm) and 6'-pyrrolidinyl-luciferins (644 nm), when compared to other beetle luciferases, revealed a larger luciferin phenolate binding pocket. The size and orientation of the side-chains of L/I/H348 are critical for amino-analogues accommodation and modulate bioluminescence color, affecting the interactions and mobility of excited oxyluciferin phenolate. The PxRE luciferase and 6'-aminoluciferins provide potential far-red combinations for bioimaging applications.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Wood KV. The chemical mechanism and evolutionary development of beetle bioluminescence. Photochem. Photobiol. Sci. 1995;62:662–673. doi: 10.1111/j.1751-1097.1995.tb08714.x. - DOI
-
- Roda A, Guardigli M, Michelini E, Mirasoli M. Bioluminescence in analytical chemistry and in vivo imaging. Trends Anal. Chem. 2009;28:307–322. doi: 10.1016/j.trac.2008.11.015. - DOI
-
- Asai, T., Nishi, S., Nakajima, Y. & Ohmiya, Y. Beetle luciferases in measuring gene expression and imaging cell functions in Luciferases and Fluorescent Proteins: Principles and Advances in Biotechnology and Bioimaging (eds Viviani, V. R. & Ohmiya, Y.) 151–160 (Transworld Research Network, 2007).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

