Effectiveness and Safety of Switching Originator and Biosimilar Epoetins in Patients with Chronic Kidney Disease in a Large-Scale Italian Cohort Study
- PMID: 31228010
- PMCID: PMC6858470
- DOI: 10.1007/s40264-019-00845-y
Effectiveness and Safety of Switching Originator and Biosimilar Epoetins in Patients with Chronic Kidney Disease in a Large-Scale Italian Cohort Study
Abstract
Introduction: Real-world data on the comparative effectiveness and safety of switching among different epoetins (including originators and biosimilars) are limited. In light of current debate about interchangeability, prescribers, some patient groups and decision makers are calling for additional post-marketing evidence on the clinical effects of switching between originator and biosimilar epoetins in chronic kidney disease (CKD) patients.
Objective: The objective of this study was to evaluate the effectiveness and safety of switching versus non-switching and of switching from originator/biosimilar epoetin alpha (ESA α) to any other epoetin in CKD patients.
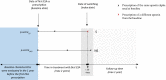
Methods: An observational, record-linkage, multi-database, retrospective cohort study was carried out in four Italian geographical areas. All subjects with at least one ESA α dispensing between 1 January 2009 and 31 December 2015 were retrieved. Switching was defined as any transition between originator/biosimilar ESA α to any other epoetin in a series of two consecutive prescriptions up to 2 years. Switchers were matched 1:1 with non-switchers by baseline propensity score and by duration of ESA α treatment. Switchers and non-switchers were followed up from switching date to a maximum of 1 year. Lack of effectiveness and safety of switching versus non-switching were evaluated through Cox regression models (hazard ratio [HR], 95% confidence interval [CI]). A direct comparison between the two switcher categories (switchers from originator/biosimilar ESA α to any other epoetin) was also performed.
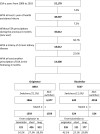
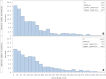
Results: Overall, 14,400 incident users of ESA α for anaemia due to CKD (61.4% originator, 38.6% biosimilar) were available for analysis. During the follow-up, we found no differences on effectiveness (HR 1.02, 95% CI 0.79-1.31 originators; HR 1.16, 95% CI 0.75-1.79 biosimilars) and safety outcomes (HR 1.08, 95% CI 0.77-1.50 originators; HR 1.20, 95% CI 0.66-2.21 biosimilars) between switchers and non-switchers of ESA α. Cumulative probabilities of recording an adverse event, either in terms of lack of effectiveness or safety issue, were the same for two switching categories CONCLUSIONS: In this large-scale Italian observational multi-database study, switching versus non-switching as well as switching from biosimilar/originator ESA α to any other epoetin in CKD patients is not associated with any effectiveness and safety outcomes.
Conflict of interest statement
Rosa Gini discloses a personal interest in sustainability of universal healthcare systems. Valeria Belleudi, Francesco Trotta, Antonio Addis, Ylenia Ingrasciotta, Valentina Ientile, Michele Tari, Maurizio Pastorello, Salvatore Scondotto, Pasquale Cananzi, Giuseppe Traversa, Marina Davoli and Gianluca Trifirò declare no conflict of interest.
Figures


References
-
- Associazione Italiana di Oncologia Medica. Linee Guida Gestione della tossicità ematopoietica in oncologia Milan; 2017. http://media.aiom.it/userfiles/files/doc/LG/2017_LGAIOM_Toss_ematopoieti.... Accessed 25 Jan 2018.
-
- Kidney Disease: Improving Global Outcomes (KDIGO) Anemia WorkGroup. KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl. 2012;2(4). https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline.... Accessed 25 Jan 2018.
-
- Sistema Nazionale Linee Guida -Istituto Superiore di Sanità, Società Italiana di Nefrologia, Ministero della Salute. Linea Guida “Identificazione, prevenzione e gestione della Malattia Renale Cronica nell’adulto”. 2015. https://www.fadoi.org/wp-content/uploads/2017/05/lineeguida-Malattia-ren.... Accessed 25 Jan 2018.
-
- Rizzo JD, Brouwers M, Hurley P, Seidenfeld J, Arcasoy MO, Spivak JL, et al. American Society of Clinical Oncology, American Society of Hematology. American Society of Clinical Oncology/American Society of Hematology clinical practice guideline update on the use of epoetin and darbepoetin in adult patients with cancer. J Clin Oncol. 2010;28(33):4996–5010. doi: 10.1200/JCO.2010.29.2201. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

