"Smartphone Medication Adherence Saves Kidneys" for Kidney Transplantation Recipients: Protocol for a Randomized Controlled Trial
- PMID: 31228175
- PMCID: PMC6611329
- DOI: 10.2196/13351
"Smartphone Medication Adherence Saves Kidneys" for Kidney Transplantation Recipients: Protocol for a Randomized Controlled Trial
Abstract
Background: Kidney transplant recipients' poor medication adherence and poor control of comorbidities, particularly hypertension, are risk factors for graft rejection, graft loss, and death. Few randomized controlled trials (RCTs) have been successful in improving sustained medication adherence and blood pressure control among kidney transplantation recipients. We provide rationale for an RCT evaluating a mobile health medical self-management system for kidney transplantation recipients called Smartphone Medication Adherence Saves Kidneys (SMASK).
Objective: Our objective is to determine whether SMASK is efficacious in improving medication adherence and sustaining blood pressure control among kidney transplantation recipients with uncontrolled hypertension and poor medication adherence compared to an enhanced standard care.
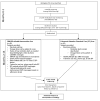
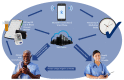
Methods: This two-arm, 6-month, phase II single-site efficacy RCT will involve 80 kidney transplantation recipients. Participants will be randomly assigned to the SMASK intervention arm or control arm. SMASK includes multilevel components: automated reminders from an electronic medication tray; tailored text messages and motivational feedback, guided by the self-determination theory; and automated summary reports for providers. Evaluations will be conducted preintervention, at 3 and 6 months, and posttrial at 12 months. Specific aims are to test the hypotheses that compared to standard care, the SMASK cohort will demonstrate significantly improved changes at 3, 6, and 12 months in the primary outcome variables medication adherence (proportion with electronic monitor-derived score >0.90) and blood pressure control (proportion meeting and sustaining adherence to the Kidney Disease Improving Global Outcomes [KDIGO] guidelines for blood pressure control); the secondary outcome variables provider adherence to KDIGO guidelines, measured by timing of medication changes and changes in self-determination theory constructs; and the exploratory outcome variables estimated glomerular filtration rate, variability in calcineurin inhibitor trough levels, and proportion of patients meeting and sustaining the 24-hour ambulatory blood pressure below 130/80 mm Hg. After the 6-month evaluation, interviews with a random sample of SMASK subjects (n=20) and health care providers (n=3-5) will assess user reactions including acceptability, usability, and aids/barriers to sustainability. Data from the RCT and interviews will be triangulated to further refine and optimize SMASK and prepare for a multisite effectiveness RCT.
Results: The SMASK project received funding from National Institute of Diabetes and Digestive and Kidney Diseases in June 2016, obtained institutional review board approval in April 2016, and began data collection in July 2016. As of July 2018, we completed enrollment with a total of 80 participants.
Conclusions: This study will provide data regarding the efficacy of SMASK to improve medication adherence and blood pressure control in a cohort of hypertensive kidney transplant recipients. An efficacious SMASK intervention will pave the way for a larger, multicenter, effectiveness RCT powered sufficiently to evaluate clinical events in a real-world setting and with the potential to demonstrate improved outcomes at lower cost than standard care.
International registered report identifier (irrid): DERR1-10.2196/13351.
Keywords: digital health; kidney transplant; mHealth; medication adherence.
©John McGillicuddy, Jessica Chandler, Luke Sox, Martina Mueller, Lynne Nemeth, Prabhakar Baliga, Frank Treiber. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 21.06.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- United States Renal Data System. 2017. Jul 23, [2019-06-03]. USRDS 2017 Annual Data Report https://www.usrds.org/adr.htm .
-
- OPTN Organ Procurement and Transplant Network. 2018. Jul 23, [2019-06-03]. http://optn.transplant.hrsa.gov/
-
- Manninen DL, Evans RW, Dugan MK. Work disability, functional limitations, and the health status of kidney transplantation recipients posttransplant. Clin Transpl. 1991:193–203. - PubMed
-
- Laupacis A, Keown P, Pus N, Krueger H, Ferguson B, Wong C, Muirhead N. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996 Jul;50(1):235–42. https://linkinghub.elsevier.com/retrieve/pii/S0085-2538(15)59601-4 S0085-2538(15)59601-4 - PubMed
-
- Wolfe RA, Ashby VB, Milford EL, Ojo AO, Ettenger RE, Agodoa LY, Held PJ, Port FK. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999 Dec 02;341(23):1725–30. doi: 10.1056/NEJM199912023412303. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

