Mobile Health Management Platform-Based Pulmonary Rehabilitation for Patients With Non-Small Cell Lung Cancer: Prospective Clinical Trial
- PMID: 31228180
- PMCID: PMC6611149
- DOI: 10.2196/12645
Mobile Health Management Platform-Based Pulmonary Rehabilitation for Patients With Non-Small Cell Lung Cancer: Prospective Clinical Trial
Abstract
Background: Lung cancer patients experience various symptoms during treatment. Although pulmonary rehabilitation is an effective way to improve these symptoms, a medical environment of limited availability makes it difficult to provide seamless and adequate rehabilitation for lung cancer patients.
Objective: This study aimed to investigate the effects of a personalized pulmonary rehabilitation program using real-time mobile patient health data for patients with non-small cell lung cancer.
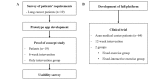
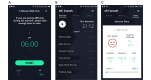
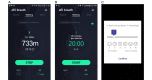
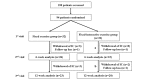
Methods: We conducted a prospective clinical trial in 64 patients with non-small cell lung cancer aged between 20 and 80 years at a large tertiary hospital in Seoul, South Korea. A 12-week personalized pulmonary rehabilitation program, called efil breath, was administered to determine the effectiveness of the newly developed rehabilitation app. Participants were randomly allocated to the fixed exercise or fixed-interactive exercise group (which received the personalized program). We measured changes in 6-minute walk distance (6MWD) and dyspnea (modified Medical Research Council [mMRC] score) at 6 weeks; and quality of life and service satisfaction at 12 weeks. We used the paired t test to analyze the variables.
Results: Patients used the newly developed mobile health pulmonary rehabilitation app and a real-time patient monitoring website. In all participants, significant changes were observed in 6MWD at 12 weeks from a mean of 433.43m (SD 65.60) to 471.25m (SD 75.69; P=.001), and mMRC from a mean score of 0.94 (0.66) to 0.61 (SD 0.82; P=.02). The intervention significantly improved their quality of life (EuroQol-visual analog scale [EQ-VAS]) compared with baseline (mean score 76.05, SD 12.37 vs 82.09, SD 13.67, respectively; P=.002).
Conclusions: A personalized mobile health-based pulmonary rehabilitation app for recording and monitoring real-time health data of patients with non-small cell lung cancer can supplement traditional health care center-based rehabilitation programs. This technology can encourage improvement of physical activity, dyspnea, and quality of life.
Keywords: carcinoma, non-small-cell lung; lung cancer; mHealth; pulmonary rehabilitation; telemedicine; telerehabilitation.
©Wonjun Ji, Hee Kwon, Sungin Lee, Seulgi Kim, Jeong Sook Hong, Yu Rang Park, Hyeong Ryul Kim, Jae Cheol Lee, Eun Ji Jung, Donghyun Kim, Chang-Min Choi. Originally published in JMIR Mhealth and Uhealth (http://mhealth.jmir.org), 21.06.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Cheville AL, Novotny PJ, Sloan JA, Basford JR, Wampfler JA, Garces YI, Jatoi A, Yang P. Fatigue, dyspnea, and cough comprise a persistent symptom cluster up to five years after diagnosis with lung cancer. J Pain Symptom Manage. 2011 Aug;42(2):202–12. doi: 10.1016/j.jpainsymman.2010.10.257. http://europepmc.org/abstract/MED/21398090 - DOI - PMC - PubMed
-
- Koczywas M, Williams AC, Cristea M, Reckamp K, Grannis FW, Tiep BL, Uman G, Ferrell B. Longitudinal changes in function, symptom burden, and quality of life in patients with early-stage lung cancer. Ann Surg Oncol. 2013 Jun;20(6):1788–97. doi: 10.1245/s10434-012-2741-4. http://europepmc.org/abstract/MED/23143593 - DOI - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

