Sexually Dimorphic Influence of Neonatal Antibiotics on Bone
- PMID: 31228216
- PMCID: PMC6886746
- DOI: 10.1002/jor.24396
Sexually Dimorphic Influence of Neonatal Antibiotics on Bone
Abstract
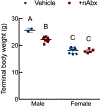
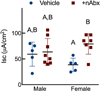
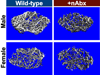
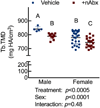
The gut microbiome (GM) contributes to host development, metabolism, and disease. Perturbations in GM composition, elicited through chronic administration of oral antibiotics (Abx) or studied using germ-free environments, alter bone mass, and microarchitecture. However, studies primarily involved chronic Abx exposure to adult mice prior to evaluating the skeletal phenotype. Children are more prone to infection with bacterial pathogens than adults and are thus treated more frequently with broad-spectrum Abx; consequently, Abx treatment disproportionately occurs during periods of greatest skeletal plasticity to anabolic cues. Because early-life exposures may exert long-lasting effects on adult health, we hypothesized that acute Abx administration during a developmentally sensitive period would elicit lasting effects on the skeletal phenotype. To test this hypothesis, neonatal mice were treated with Abx (P7-P23; oral gavage) or vehicle (water); GM composition, gut physiology, and bone structural and material properties were assessed in adulthood (8 weeks). We found sexually dimorphic effects of neonatal Abx administration on GM composition, gut barrier permeability, and the skeleton, indicating a negative role for neonatal Abx on bone mass and quality. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 37:2122-2129, 2019.
Keywords: antibiotics; bone; gut microbiota; intestinal barrier.
© 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures