Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation
- PMID: 31228465
- PMCID: PMC6733644
- DOI: 10.1016/j.bcp.2019.06.017
Enterobactin, an iron chelating bacterial siderophore, arrests cancer cell proliferation
Abstract
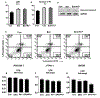
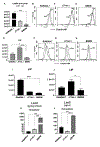
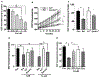
Iron is essential for many biological functions, including being a cofactor for enzymes involved in cell proliferation. In line, it has been shown that cancer cells can perturb their iron metabolism towards retaining an abundant iron supply for growth and survival. Accordingly, it has been suggested that iron deprivation through the use of iron chelators could attenuate cancer progression. While they have exhibited anti-tumor properties in vitro, the current therapeutic iron chelators are inadequate due to their low efficacy. Therefore, we investigated whether the bacterial catecholate-type siderophore, enterobactin (Ent), could be used as a potent anti-cancer agent given its strong iron chelation property. We demonstrated that iron-free Ent can exert cytotoxic effects specifically towards monocyte-related tumor cell lines (RAW264.7 and J774A.1), but not primary cells, i.e. bone marrow-derived macrophages (BMDMs), through two mechanisms. First, we observed that RAW264.7 and J774A.1 cells preserve a bountiful intracellular labile iron pool (LIP), whose homeostasis can be disrupted by Ent. This may be due, in part, to the lower levels of lipocalin 2 (Lcn2; an Ent-binding protein) in these cell lines, whereas the higher levels of Lcn2 in BMDMs could prevent Ent from hindering their LIP. Secondly, we observed that Ent could dose-dependently impede reactive oxygen species (ROS) generation in the mitochondria. Such disruption in LIP balance and mitochondrial function may in turn promote cancer cell apoptosis. Collectively, our study highlights Ent as an anti-cancer siderophore, which can be exploited as an unique agent for cancer therapy.
Keywords: Deferoxamine; Enterochelin; Labile iron pool; Lipocalin 2; Mitochondrial respiration.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Figures


References
-
- Baudino TA, Targeted Cancer Therapy: The Next Generation of Cancer Treatment, Curr Drug Discov Technol 12(1) (2015) 3–20. - PubMed
-
- Urruticoechea A, Alemany R, Balart J, Villanueva A, Vinals F, Capella G, Recent advances in cancer therapy: an overview, Curr Pharm Des 16(1) (2010) 3–10. - PubMed
-
- Richardson DR, Ponka P, The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells, Biochim Biophys Acta 1331(1) (1997) 1–40. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

