Generation of Human iPSC-Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction
- PMID: 31228606
- PMCID: PMC6722387
- DOI: 10.1016/j.jcmgh.2019.06.004
Generation of Human iPSC-Derived Intestinal Epithelial Cell Monolayers by CDX2 Transduction
Abstract
Background & aims: To develop an effective and safe orally administered drug, it is important to predict its intestinal absorption rate, intestinal first-pass effect, and drug-drug interactions of orally administered drugs. However, there is no existing model to comprehensively predict the intestinal pharmacokinetics and drug-response of orally administered drugs. In this study, we attempted to generate homogenous and functional intestinal epithelial cells from human induced pluripotent stem (iPS) cells for pharmaceutical research.
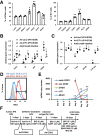
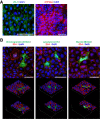
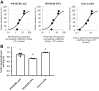
Methods: We generated almost-homogenous Villin- and zonula occludens-1 (ZO1)-positive intestinal epithelial cells by caudal-related homeobox transcription factor 2 (CDX2) transduction into human iPS cell-derived intestinal progenitor cells.
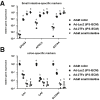
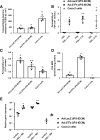
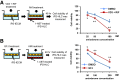
Results: The drug absorption rates in human iPS cell-derived intestinal epithelial cell monolayers (iPS-IECM) were highly correlated with those in humans (R2=0.91). The expression levels of cytochrome P450 (CYP) 3A4, a dominant drug-metabolizing enzyme in the small intestine, in human iPS-IECM were similar to those in human small intestine in vivo. In addition, intestinal availability in human iPS-IECM (the fraction passing the gut wall: Fg=0.73) was more similar to that in the human small intestine in vivo (Fg=0.57) than to that in Caco-2 cells (Fg=0.99), a human colorectal adenocarcinoma cell line. Moreover, the drug-drug interaction and drug-food interaction could be observed by using our human iPS-IECM in the presence of an inducer and inhibitor of CYP3A4, i.e., rifampicin and grape fruit juice, respectively.
Conclusion: Taking these results together, we succeeded in generating the human iPS-IECM that can be applied to various intestinal pharmacokinetics and drug-response tests of orally administered drugs.
Keywords: Adenovirus; CYP3A4; Differentiation; Intestinal First-Pass Effect.
Copyright © 2019 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Paine M.F., Khalighi M., Fisher J.M., Shen D.D., Kunze K.L., Marsh C.L., Perkins J.D., Thummel K.E. Characterization of interintestinal and intraintestinal variations in human CYP3A-dependent metabolism. J Pharmacol Exp Ther. 1997;283:1552–1562. - PubMed
-
- Thummel K.E., Kunze K.L., Shen D.D. Enzyme-catalyzed processes of first-pass hepatic and intestinal drug extraction. Adv Drug Deliv Rev. 1997;27:99–127. - PubMed
-
- Fromm M.F., Busse D., Kroemer H.K., Eichelbaum M. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology. 1996;24:796–801. - PubMed
-
- Gomez D.Y., Wacher V.J., Tomlanovich S.J., Hebert M.F., Benet L.Z. The effects of ketoconazole on the intestinal metabolism and bioavailability of cyclosporine. Clin Pharm Ther. 1995;58:15–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

