Effects of glassing matrix deuteration on the relaxation properties of hyperpolarized 13C spins and free radical electrons at cryogenic temperatures
- PMID: 31228902
- PMCID: PMC6588520
- DOI: 10.1063/1.5096036
Effects of glassing matrix deuteration on the relaxation properties of hyperpolarized 13C spins and free radical electrons at cryogenic temperatures
Abstract
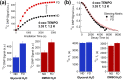
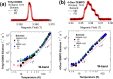
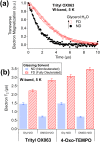
Glassing matrix deuteration could be a beneficial sample preparation method for 13C dynamic nuclear polarization (DNP) when large electron paramagnetic resonance (EPR) width free radicals are used. However, it could yield the opposite DNP effect when samples are doped with small EPR width free radicals. Herein, we have investigated the influence of solvent deuteration on the 13C nuclear and electron relaxation that go along with the effects on 13C DNP intensities at 3.35 T and 1.2 K. For 13C DNP samples doped with trityl OX063, the 13C DNP signals decreased significantly when the protons are replaced by deuterons in glycerol:water or DMSO:water solvents. Meanwhile, the corresponding solid-state 13C T1 relaxation times of trityl OX063-doped samples generally increased upon solvent deuteration. On the other hand, 13C DNP signals improved by a factor of ∼1.5 to 2 upon solvent deuteration of samples doped with 4-oxo-TEMPO. Despite this 13C DNP increase, there were no significant differences recorded in 13C T1 values of TEMPO-doped samples with nondeuterated or fully deuterated glassing matrices. While solvent deuteration appears to have a negligible effect on the electron T1 relaxation of both free radicals, the electron T2 relaxation times of these two free radicals generally increased upon solvent deuteration. These overall results suggest that while the solid-phase 13C DNP signals are dependent upon the changes in total nuclear Zeeman heat capacity, the 13C relaxation effects are related to 2H/1H nuclear spin diffusion-assisted 13C polarization leakage in addition to the dominant paramagnetic relaxation contribution of free radical centers.
Figures


Similar articles
-
Influence of 13C Isotopic Labeling Location on Dynamic Nuclear Polarization of Acetate.J Phys Chem A. 2017 May 4;121(17):3227-3233. doi: 10.1021/acs.jpca.7b01844. Epub 2017 Apr 24. J Phys Chem A. 2017. PMID: 28422500 Free PMC article.
-
Influence of deuteration in the glassing matrix on 13C dynamic nuclear polarization.Phys Chem Chem Phys. 2013 May 21;15(19):7032-5. doi: 10.1039/c3cp50750e. Phys Chem Chem Phys. 2013. PMID: 23552448 Free PMC article.
-
Impact of Gd3+ on DNP of [1-13C]pyruvate doped with trityl OX063, BDPA, or 4-oxo-TEMPO.J Phys Chem A. 2012 May 31;116(21):5129-38. doi: 10.1021/jp302399f. Epub 2012 May 18. J Phys Chem A. 2012. PMID: 22571288 Free PMC article.
-
Impact of Ho(3+)-doping on (13)C dynamic nuclear polarization using trityl OX063 free radical.Phys Chem Chem Phys. 2016 Aug 21;18(31):21351-9. doi: 10.1039/c6cp03954e. Epub 2016 Jul 18. Phys Chem Chem Phys. 2016. PMID: 27424954 Free PMC article.
-
Dynamic nuclear polarization of carbonyl and methyl 13C spins of acetate using 4-oxo-TEMPO free radical.J Chem Phys. 2018 Aug 7;149(5):054302. doi: 10.1063/1.5043378. J Chem Phys. 2018. PMID: 30089385
Cited by
-
Directly Bound Deuterons Increase X-Nuclei Hyperpolarization using Dynamic Nuclear Polarization.Chemphyschem. 2023 Sep 15;24(18):e202300144. doi: 10.1002/cphc.202300144. Epub 2023 Jul 24. Chemphyschem. 2023. PMID: 37431622 Free PMC article.
References
-
- Levitt M. H., Spin Dynamics: Basics of Nuclear Magnetic Resonance, 2nd ed. (John Wiley & Sons, Ltd., West Sussex, 2008).
-
- Slichter C. P., Principles of Magnetic Resonance, 3rd ed. (Springer-Verlag, Berlin, 1990).
-
- Abragam A., The Principles of Nuclear Magnetism (Clarendon Press, Oxford, 1961).
-
- Abragam A. and Goldman M., Rep. Prog. Phys. 41, 395 (1978).10.1088/0034-4885/41/3/002 - DOI
-
- Crabb D. G. and Meyer W., Annu. Rev. Nucl. Part. Sci. 47, 67 (1997).10.1146/annurev.nucl.47.1.67 - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

