Membrane and nuclear initiated estrogenic regulation of homeostasis
- PMID: 31229508
- PMCID: PMC6923613
- DOI: 10.1016/j.steroids.2019.108428
Membrane and nuclear initiated estrogenic regulation of homeostasis
Abstract
Reproduction and energy balance are inextricably linked in order to optimize the evolutionary fitness of an organism. With insufficient or excessive energy stores a female is liable to suffer complications during pregnancy and produce unhealthy or obesity-prone offspring. The quintessential function of the hypothalamus is to act as a bridge between the endocrine and nervous systems, coordinating fertility and autonomic functions. Across the female reproductive cycle various motivations wax and wane, following levels of ovarian hormones. Estrogens, more specifically 17β-estradiol (E2), coordinate a triumvirate of hypothalamic neurons within the arcuate nucleus (ARH) that govern the physiological underpinnings of these behavioral dynamics. Arising from a common progenitor pool of cells, this triumvirate is composed of the kisspeptin (Kiss1ARH), proopiomelanocortin (POMC), and neuropeptide Y/agouti-related peptide (AgRP) neurons. Although the excitability of these neuronal subpopulations is subject to genomic and rapid estrogenic regulation, kisspeptin neurons are the most sensitive, reflecting their integral function in female fertility. Based on the premise that E2 coordinates autonomic functions around reproduction, we will review the recent findings on the synaptic interactions between Kiss1, AgRP and POMC neurons and how the rapid membrane-initiated and intracellular signaling cascades activated by E2 in these neurons are critical for control of homeostatic functions supporting reproduction.
Keywords: Hypothalamus; Kisspeptin neurons; Neuropeptide Y/agouti-related peptide neurons; Peptides; Proopiomelanocortin neurons; Synaptic transmission.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures

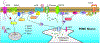
Similar articles
-
Estrogenic regulation of reproduction and energy homeostasis by a triumvirate of hypothalamic arcuate neurons.J Neuroendocrinol. 2022 Jun;34(6):e13145. doi: 10.1111/jne.13145. Epub 2022 May 17. J Neuroendocrinol. 2022. PMID: 35581942 Free PMC article. Review.
-
Deletion of Stim1 in Hypothalamic Arcuate Nucleus Kiss1 Neurons Potentiates Synchronous GCaMP Activity and Protects against Diet-Induced Obesity.J Neurosci. 2021 Nov 24;41(47):9688-9701. doi: 10.1523/JNEUROSCI.0622-21.2021. Epub 2021 Oct 15. J Neurosci. 2021. PMID: 34654752 Free PMC article.
-
Arcuate Kisspeptin Neurons Coordinate Reproductive Activities with Metabolism.Semin Reprod Med. 2019 May;37(3):131-140. doi: 10.1055/s-0039-3400251. Epub 2019 Dec 23. Semin Reprod Med. 2019. PMID: 31869841 Free PMC article. Review.
-
AgRP to Kiss1 neuron signaling links nutritional state and fertility.Proc Natl Acad Sci U S A. 2017 Feb 28;114(9):2413-2418. doi: 10.1073/pnas.1621065114. Epub 2017 Feb 14. Proc Natl Acad Sci U S A. 2017. PMID: 28196880 Free PMC article.
-
Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons.Horm Behav. 2018 Aug;104:146-155. doi: 10.1016/j.yhbeh.2018.04.001. Epub 2018 Apr 21. Horm Behav. 2018. PMID: 29626486 Free PMC article. Review.
Cited by
-
Hypothalamic Estrogen Receptor α Is Essential for Female Marmoset Sexual Behavior Without Protecting From Obesity.J Endocr Soc. 2025 Feb 5;9(3):bvaf012. doi: 10.1210/jendso/bvaf012. eCollection 2025 Feb 4. J Endocr Soc. 2025. PMID: 39911518 Free PMC article.
References
-
- King JC, Tobet SA, Snavely FL, and Arimura AA, The LHRH system in normal and neonatally androgenized female rats. Peptides, 1980. 1: p. 85–100.
-
- Lehman MN, Robinson JE, Karsch FJ, and Silverman AJ, Immunocytochemical localization of luteinizing hormone-releasing hormone(LHRH) pathways in the sheep brain during anestrous and the mid-luteal phase of the estrous cycle. The Journal of Comparative Neurology, 1986. 244: p. 19–35. - PubMed
-
- Silverman AJ, Distribution of luteinizing hormone-releasing hormone (LH-RH) in the guinea pig brain. Endocrinology, 1976. 99: p. 30–41. - PubMed
-
- Silverman AJ, Antunes JL, Abrams GM, Nilaver G, Thau R, Robinson JA, Ferin M, and Krey LC, The luteinizing hormone-releasing hormone pathway in rhesus(Macaca mulatta) and pigtailed (Macaca nemestrina) monkeys: New observations on thick, unembedded sections. The Journal of Comparative Neurology, 1982. 211: p. 309–317. - PubMed
-
- King JC, Anthony ELP, Fitzgerald DM, and Stopa EG, Luteinizing hormone-releasing hormone neurons in human preoptic/hypothalamus: differential intraneuronal localization of immunoreactive forms. The Journal of Clinical Endocrinology & Metabolism, 1985. 60: p. 88–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

