Discoidin domain receptors: Micro insights into macro assemblies
- PMID: 31229648
- PMCID: PMC6726542
- DOI: 10.1016/j.bbamcr.2019.06.010
Discoidin domain receptors: Micro insights into macro assemblies
Abstract
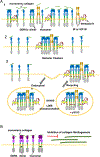
Assembly of cell-surface receptors into specific oligomeric states and/or clusters before and after ligand binding is an important feature governing their biological function. Receptor oligomerization can be mediated by specific domains of the receptor, ligand binding, configurational changes or other interacting molecules. In this review we summarize our understanding of the oligomeric state of discoidin domain receptors (DDR1 and DDR2), which belong to the receptor tyrosine kinase family (RTK). DDRs form an interesting system from an oligomerization perspective as their ligand collagen(s) can also undergo supramolecular assembly to form fibrils. Even though DDR1 and DDR2 differ in the domains responsible to form ligand-free dimers they share similarities in binding to soluble, monomeric collagen. However, only DDR1b forms globular clusters in response to monomeric collagen and not DDR2. Interestingly, both DDR1 and DDR2 are assembled into linear clusters by the collagen fibril. Formation of these clusters is important for receptor phosphorylation and is mediated in part by other membrane components. We summarize how the oligomeric status of DDRs shares similarities with other members of the RTK family and with collagen receptors. Unraveling the multiple macro-molecular configurations adopted by this receptor-ligand pair can provide novel insights into the intricacies of cell-matrix interactions.
Keywords: Cluster; Collagen; Dimer; Discoidin; Oligomer; Receptor tyrosine kinases.
Copyright © 2019 Elsevier B.V. All rights reserved.
Figures



Similar articles
-
Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I.J Mol Biol. 2019 Jan 18;431(2):368-390. doi: 10.1016/j.jmb.2018.11.015. Epub 2018 Nov 17. J Mol Biol. 2019. PMID: 30458172 Free PMC article.
-
A structural prospective for collagen receptors such as DDR and their binding of the collagen fibril.Biochim Biophys Acta Mol Cell Res. 2019 Nov;1866(11):118478. doi: 10.1016/j.bbamcr.2019.04.008. Epub 2019 Apr 18. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 31004686 Review.
-
Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2.J Biol Chem. 2003 May 9;278(19):16761-9. doi: 10.1074/jbc.M301370200. Epub 2003 Feb 28. J Biol Chem. 2003. PMID: 12611880
-
Using synthetic peptides and recombinant collagen to understand DDR-collagen interactions.Biochim Biophys Acta Mol Cell Res. 2019 Nov;1866(11):118458. doi: 10.1016/j.bbamcr.2019.03.005. Epub 2019 Mar 15. Biochim Biophys Acta Mol Cell Res. 2019. PMID: 30880148 Review.
-
Discoidin Domain Receptors, DDR1b and DDR2, Promote Tumour Growth within Collagen but DDR1b Suppresses Experimental Lung Metastasis in HT1080 Xenografts.Sci Rep. 2020 Feb 11;10(1):2309. doi: 10.1038/s41598-020-59028-w. Sci Rep. 2020. PMID: 32047176 Free PMC article.
Cited by
-
Receptor Tyrosine Kinases in Development: Insights from Drosophila.Int J Mol Sci. 2019 Dec 26;21(1):188. doi: 10.3390/ijms21010188. Int J Mol Sci. 2019. PMID: 31888080 Free PMC article. Review.
-
Discoidin Domain Receptor 2 Mediates Lysophosphatidic Acid-Induced Ovarian Cancer Aggressiveness.Int J Mol Sci. 2021 May 20;22(10):5374. doi: 10.3390/ijms22105374. Int J Mol Sci. 2021. PMID: 34065317 Free PMC article.
-
Investigation of Cell Mechanics and Migration on DDR2-Expressing Neuroblastoma Cell Line.Life (Basel). 2024 Oct 2;14(10):1260. doi: 10.3390/life14101260. Life (Basel). 2024. PMID: 39459560 Free PMC article.
-
The Na+-K+-ATPase alpha subunit is an entry receptor for white spot syndrome virus.mBio. 2025 Mar 12;16(3):e0378724. doi: 10.1128/mbio.03787-24. Epub 2025 Feb 18. mBio. 2025. PMID: 39964166 Free PMC article.
-
Discoidin domain receptors; an ancient family of collagen receptors has major roles in bone development, regeneration and metabolism.Front Dent Med. 2023;4:1181817. doi: 10.3389/fdmed.2023.1181817. Epub 2023 May 11. Front Dent Med. 2023. PMID: 38222874 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

