Observational study to determine the utility of hospital administrative data to support case finding of English patients at higher risk of severe healthcare-related harm
- PMID: 31230000
- PMCID: PMC6596963
- DOI: 10.1136/bmjopen-2018-025372
Observational study to determine the utility of hospital administrative data to support case finding of English patients at higher risk of severe healthcare-related harm
Abstract
Objectives: To identify ways of using routine hospital data to improve the efficiency of retrospective reviews of case records for identifying avoidable severe harm DESIGN: Development and testing of thresholds and criteria for two indirect indicators of healthcare-related harm (long length of stay (LOS) and emergency readmission) to determine the yield of specified harms coded in Hospital Episode Statistics (HES).
Setting: Acute National Health Service hospitals in England.
Participants: HES for acute myocardial infarction (AMI), bowel cancer surgery and hip replacement admissions from 2014 to 2015.
Interventions: Case-mix-adjusted linear regression models were used to determine expected LOS. Different thresholds were examined to determine the association with harm. Screening criteria for readmission included time to readmission, length of readmission and diagnoses in initial admission and readmission. The association with harm was examined for each criterion.
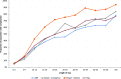
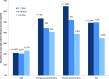
Results: The proportions of AMI cases with a harm code increased from 14% among all cases to 47% if a threshold of three times the expected LOS was used. For hip replacement the respective increase was from 10% to 51%. However as the number of patients at these higher thresholds was small, the overall proportion of harm identified is relatively small (15%, 19%, 9% and 8% among AMI, urgent bowel surgery, elective bowel surgery and hip replacement cohorts, respectively). Selection of the time to readmission had an effect on the yield of harms but this varied with condition. At least 50% of surgical patients had a harm code if readmitted within 7 days compared with 21% of patients with AMI.
Conclusions: Our approach would select a substantial number of patients for case record review. Many of these cases would contain no evidence of healthcare-related harm. In practice, Trusts may choose how many reviews it is feasible to do in advance and then select random samples of cases that satisfy the screening criteria.
Keywords: case finding; healthcare-related harm; hospital administrative data.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
Similar articles
-
Hospital Readmission Rates Following AMI: Potential Interventions to Improve Efficiency.South Med J. 2018 Feb;111(2):93-97. doi: 10.14423/SMJ.0000000000000768. South Med J. 2018. PMID: 29394425
-
National trends in emergency readmission rates: a longitudinal analysis of administrative data for England between 2006 and 2016.BMJ Open. 2018 Mar 12;8(3):e020325. doi: 10.1136/bmjopen-2017-020325. BMJ Open. 2018. PMID: 29530912 Free PMC article.
-
The effects of surgical volumes and training centre status on outcomes following total joint replacement: analysis of the Hospital Episode Statistics for England.J Public Health (Oxf). 2006 Jun;28(2):116-24. doi: 10.1093/pubmed/fdl003. Epub 2006 Apr 5. J Public Health (Oxf). 2006. PMID: 16597628
-
Mortality, readmission and length of stay have different relationships using hospital-level versus patient-level data: an example of the ecological fallacy affecting hospital performance indicators.BMJ Qual Saf. 2018 Jun;27(6):474-483. doi: 10.1136/bmjqs-2017-006776. Epub 2017 Oct 6. BMJ Qual Saf. 2018. PMID: 28986516
-
Factors associated with hospital stay length, discharge destination, and 30-day readmission rate after primary hip or knee arthroplasty: Retrospective Cohort Study.Orthop Traumatol Surg Res. 2019 Sep;105(5):949-955. doi: 10.1016/j.otsr.2019.04.012. Epub 2019 Jun 15. Orthop Traumatol Surg Res. 2019. PMID: 31208932 Review.
References
-
- Australian Institute for Health and Welfare. Reporting of adverse event in routinely collected data sets in Australia: Australian Institute for Health and Welfare, 2001.
-
- Davis P, Lay-Yee R, Briant R, et al. . Adverse events in New Zealand public hospitals I: occurrence and impact. N Z Med J 2002;115:U271. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical