Inhibition of Muscular Nociceptive Afferents via the Activation of Cutaneous Nociceptors in a Rat Model of Inflammatory Muscle Pain
- PMID: 31230211
- PMCID: PMC6940416
- DOI: 10.1007/s12264-019-00406-4
Inhibition of Muscular Nociceptive Afferents via the Activation of Cutaneous Nociceptors in a Rat Model of Inflammatory Muscle Pain
Abstract
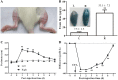
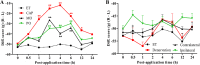
Topical irritants such as capsaicin (CAP), peppermint oil (PO), and mustard oil (MO) are effective in relieving inflammatory muscle pain. We investigated the effects of topical irritants in a rat model of inflammatory muscle pain produced by injecting complete Freund's adjuvant (CFA) into the tibialis anterior muscle. CFA-induced mechanical hypersensitivity and the spontaneous activity of muscular nociceptive afferents, and decreased weight-bearing of the hindlimb were relieved by topical application of CAP, PO, or MO on the skin overlying the inflamed muscle. The effects of topical irritants were abolished when applied to the skin on the ipsilateral plantar region or on the contralateral leg, or when the relevant cutaneous nerve or dorsal root was transected. Our results demonstrated that topical irritants may alleviate inflammatory muscle pain via activating cutaneous nociceptors and subsequently inhibiting the abnormal activity of muscular nociceptive neurons.
Keywords: Capsaicin; Cutaneous nociceptor; Inflammatory muscle pain; Muscular nociceptor.
Conflict of interest statement
All authors claim that there are no conflicts of interest.
Figures



Similar articles
-
Mechanisms of topical analgesics in relieving pain in an animal model of muscular inflammation.Pain Med. 2013 Sep;14(9):1381-7. doi: 10.1111/pme.12199. Epub 2013 Jul 19. Pain Med. 2013. PMID: 23870161
-
Inflammation and hyperalgesia in rats neonatally treated with capsaicin: effects on two classes of nociceptive neurons in the superficial dorsal horn.Pain. 1994 Nov;59(2):287-300. doi: 10.1016/0304-3959(94)90082-5. Pain. 1994. PMID: 7892027
-
Roles of capsaicin-sensitive primary afferents in differential rat models of inflammatory pain: a systematic comparative study in conscious rats.Exp Neurol. 2007 Mar;204(1):244-51. doi: 10.1016/j.expneurol.2006.10.011. Epub 2006 Dec 22. Exp Neurol. 2007. PMID: 17188267
-
Peripheral and central mechanisms of cutaneous hyperalgesia.Prog Neurobiol. 1992;38(4):397-421. doi: 10.1016/0301-0082(92)90027-c. Prog Neurobiol. 1992. PMID: 1574584 Review.
-
[What is a nociceptor?].Anaesthesist. 1997 Feb;46(2):142-53. doi: 10.1007/s001010050384. Anaesthesist. 1997. PMID: 9133176 Review. German.
Cited by
-
Electroacupuncture-Induced Muscular Inflammatory Pain Relief Was Associated With Activation of Low-Threshold Mechanoreceptor Neurons and Inhibition of Wide Dynamic Range Neurons in Spinal Dorsal Horn.Front Neurosci. 2021 Jul 8;15:687173. doi: 10.3389/fnins.2021.687173. eCollection 2021. Front Neurosci. 2021. PMID: 34305519 Free PMC article.
-
The Effect of Pre-Electroacupuncture on Nociceptive Discharges of Spinal Wide Dynamic Range Neurons in Rat.J Pain Res. 2023 Mar 7;16:695-706. doi: 10.2147/JPR.S396481. eCollection 2023. J Pain Res. 2023. PMID: 36915279 Free PMC article.
-
Involvement of the Transient Receptor Channels in Preclinical Models of Musculoskeletal Pain.Curr Neuropharmacol. 2024;22(1):72-87. doi: 10.2174/1570159X21666230908094159. Curr Neuropharmacol. 2024. PMID: 37694792 Free PMC article. Review.
-
Transcriptional profiles of TGF-β superfamily members in the lumbar DRGs and the effects of activins A and C on inflammatory pain in rats.J Physiol Biochem. 2023 May;79(2):313-325. doi: 10.1007/s13105-022-00943-z. Epub 2023 Jan 25. J Physiol Biochem. 2023. PMID: 36696051
-
Electroacupuncture and Moxibustion-Like Stimulation Relieves Inflammatory Muscle Pain by Activating Local Distinct Layer Somatosensory Afferent Fibers.Front Neurosci. 2021 Jul 15;15:695152. doi: 10.3389/fnins.2021.695152. eCollection 2021. Front Neurosci. 2021. PMID: 34335169 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

