Chemical modulation of apoptosis in molluscan cell cultures
- PMID: 31230213
- PMCID: PMC6717236
- DOI: 10.1007/s12192-019-01014-x
Chemical modulation of apoptosis in molluscan cell cultures
Abstract
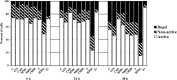
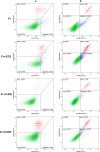
This study focused on the alterations that occur in larval molluscan cells after administration of apoptotic inducers and inhibitors used in mammalian cells in response to cold stress. This is the first report on apoptosis modulation in molluscan cells assessed by flow cytometry. Mitochondrial activity, general caspase activation, and membrane integrity of control molluscan cells were compared to those processes in frozen-thawed molluscan cells, primary mouse embryonic fibroblasts, and human colon tumor cells prior to treatment and after incubation with apoptotic inducers or inhibitors. We tested three apoptotic inducers (staurosporine, camptothecin, and mitomycin C, routinely used for the chemical induction of apoptosis in different mammalian cells) and found that only staurosporine resulted in an evident apoptotic increase in molluscan cell cultures: 9.06% early apoptotic cells in comparison with 5.63% in control frozen-thawed cells and 20.6% late apoptotic cells in comparison with 10.68% in controls. Camptothecin did not significantly induce molluscan cell apoptosis but did cause a slight increase in the number of active cells after thawing. Mitomycin C produced similar results, but its effect was less pronounced. In addition, we hypothesize that the use of the apoptotic inhibitors could reduce apoptosis, which is significant after cryopreservation in molluscan cells; however, our attempts failed. Development in this direction is important for understanding the mechanisms of marine organisms' cold susceptibility.
Keywords: Apoptotic inducers; Apoptotic inhibitors; Cell death pathways; Flow cytometry; Mussel; Mytilus trossulus.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


Similar articles
-
Chemical-induced apoptotic cell death in tomato cells: involvement of caspase-like proteases.Planta. 2000 Oct;211(5):656-62. doi: 10.1007/s004250000341. Planta. 2000. PMID: 11089678
-
DDX3 regulates DNA damage-induced apoptosis and p53 stabilization.Biochim Biophys Acta. 2013 Jun;1833(6):1489-97. doi: 10.1016/j.bbamcr.2013.02.026. Epub 2013 Mar 5. Biochim Biophys Acta. 2013. PMID: 23470959 Free PMC article.
-
Role for ionic fluxes on cell death and apoptotic volume decrease in cultured cerebellar granule neurons.Neuroscience. 2010 May 5;167(2):298-311. doi: 10.1016/j.neuroscience.2010.01.046. Epub 2010 Feb 6. Neuroscience. 2010. PMID: 20144697
-
Nitric oxide inhibits caspase activation and apoptotic morphology but does not rescue neuronal death.J Cereb Blood Flow Metab. 2005 Mar;25(3):348-57. doi: 10.1038/sj.jcbfm.9600036. J Cereb Blood Flow Metab. 2005. PMID: 15660100
-
Silencing RBBP6 (Retinoblastoma Binding Protein 6) sensitises breast cancer cells MCF7 to staurosporine and camptothecin-induced cell death.Immunobiology. 2014 Aug;219(8):593-601. doi: 10.1016/j.imbio.2014.03.002. Epub 2014 Mar 21. Immunobiology. 2014. PMID: 24703106
Cited by
-
In vitro proliferation of Mytilus edulis male germ cell progenitors.PLoS One. 2024 Feb 9;19(2):e0292205. doi: 10.1371/journal.pone.0292205. eCollection 2024. PLoS One. 2024. PMID: 38335194 Free PMC article.
-
The effects of cold stress on Mytilus species in the natural environment.Cell Stress Chaperones. 2020 Nov;25(6):821-832. doi: 10.1007/s12192-020-01109-w. Epub 2020 Apr 15. Cell Stress Chaperones. 2020. PMID: 32297161 Free PMC article. Review.
-
Status in molluscan cell line development in last one decade (2010-2020): impediments and way forward.Cytotechnology. 2022 Aug;74(4):433-457. doi: 10.1007/s10616-022-00539-x. Epub 2022 Jul 24. Cytotechnology. 2022. PMID: 36110153 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

