Selective bacterial degradation of the extracellular matrix attaching the gingiva to the tooth
- PMID: 31230388
- PMCID: PMC6771947
- DOI: 10.1111/eos.12623
Selective bacterial degradation of the extracellular matrix attaching the gingiva to the tooth
Abstract
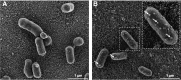
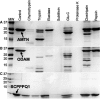
The junctional epithelium (JE) is a specialized portion of the gingiva that seals off the tooth-supporting tissues from the oral environment. This relationship is achieved via a unique adhesive extracellular matrix that is, in fact, a specialized basal lamina (sBL). Three unique proteins - amelotin (AMTN), odontogenic ameloblast-associated (ODAM), and secretory calcium-binding phosphoprotein proline-glutamine rich 1 (SCPPPQ1) - together with laminin-332 structure the supramolecular organization of this sBL and determine its adhesive capacity. Despite the constant challenge of the JE by the oral microbiome, little is known of the susceptibility of the sBL to bacterial degradation. Assays with trypsin-like proteases, as well as incubation with Porphyromonas gingivalis, Prevotella intermedia, and Treponema denticola, revealed that all constituents, except SCPPPQ1, were rapidly degraded. Porphyromonas gingivalis was also shown to alter the supramolecular network of reconstituted and native sBLs. These results provide evidence that proteolytic enzymes and selected gram-negative periodontopathogenic bacteria can attack this adhesive extracellular matrix, intimating that its degradation could contribute to progression of periodontal diseases.
Keywords: Porphyromonas gingivalis; junctional epithelium; periodontal diseases; specialized basal lamina; supramolecular network.
© 2019 The Authors. Eur J Oral Sci published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interests.
Figures





References
-
- Schroeder HE, Listgarten MA. The gingival tissues: the architecture of peridontal protection. Periodontol 2000 1997; 13: 91–120. - PubMed
-
- Bosshardt DD, Lang NP. The junctional epithelium: from health to disease. J Dent Res 2005; 84: 9–20. - PubMed
-
- Hormia M, Owaribe K, Virtanen I. The dento‐epithelial junction: cell adhesion by type I hemidesmosomes in the absence of a true basal lamina. J Periodontol 2001; 72: 788–797. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

