The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex
- PMID: 31230762
- PMCID: PMC7935470
- DOI: 10.1016/j.neuron.2019.05.037
The Autism-Associated Gene Scn2a Contributes to Dendritic Excitability and Synaptic Function in the Prefrontal Cortex
Abstract
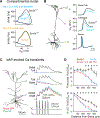
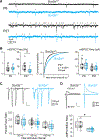
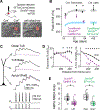
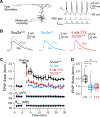
Autism spectrum disorder (ASD) is strongly associated with de novo gene mutations. One of the most commonly affected genes is SCN2A. ASD-associated SCN2A mutations impair the encoded protein NaV1.2, a sodium channel important for action potential initiation and propagation in developing excitatory cortical neurons. The link between an axonal sodium channel and ASD, a disorder typically attributed to synaptic or transcriptional dysfunction, is unclear. Here we show that NaV1.2 is unexpectedly critical for dendritic excitability and synaptic function in mature pyramidal neurons in addition to regulating early developmental axonal excitability. NaV1.2 loss reduced action potential backpropagation into dendrites, impairing synaptic plasticity and synaptic strength, even when NaV1.2 expression was disrupted in a cell-autonomous fashion late in development. These results reveal a novel dendritic function for NaV1.2, providing insight into cellular mechanisms probably underlying circuit and behavioral dysfunction in ASD.
Keywords: 2-photon imaging; NaV1.2; Scn2a; action potential backpropagation; autism spectrum disorder; dendritic excitability; electrophysiology; haploinsufficiency; intellectual disability; sodium channel; synaptic plasticity.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



Comment in
-
Loss of NaV1.2-Dependent Backpropagating Action Potentials in Dendrites Contributes to Autism and Intellectual Disability.Neuron. 2019 Aug 21;103(4):551-553. doi: 10.1016/j.neuron.2019.07.032. Neuron. 2019. PMID: 31437449
References
-
- Barth AMI, Vizi ES, Zelles T, and Lendvai B. (2008). α 2 -Adrenergic Receptors Modify Dendritic Spike Generation Via HCN Channels in the Prefrontal Cortex. J. Neurophysiol 99, 394–401. - PubMed
-
- Bean BP (2007). The action potential in mammalian central neurons. Nat. Rev. Neurosci 8, 451–465. - PubMed
-
- Beaulieu-Laroche L, and Harnett MT (2018). Dendritic Spines Prevent Synaptic Voltage Clamp. Neuron 97, 75–82.e3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

