Rapid Identification and Antimicrobial Susceptibility Testing for Urinary Tract Pathogens by Direct Analysis of Urine Samples Using a MALDI-TOF MS-Based Combined Protocol
- PMID: 31231323
- PMCID: PMC6560049
- DOI: 10.3389/fmicb.2019.01182
Rapid Identification and Antimicrobial Susceptibility Testing for Urinary Tract Pathogens by Direct Analysis of Urine Samples Using a MALDI-TOF MS-Based Combined Protocol
Abstract
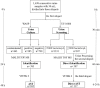
Usually, 18-48 h are needed for the identification of microbial pathogens causing urinary tract infections (UTIs) by urine culture. Moreover, antimicrobial susceptibility testing (AST) takes an additional 18-24 h. Rapid identification and AST of the pathogens allow fast and precise treatment. The objective of this study was to shorten the time of diagnosis of UTIs by combining pathogen screening through flow cytometry, microbial identification by matrix-assisted laser desorption ionisation time-of-flight mass spectrometry (MALDI-TOF MS), and AST using the VITEK 2 system for the direct analysis of urine samples. We analyzed 1,638 urine samples from patients with suspected UTIs submitted to the microbiology laboratory for culture. Each urine sample had an approximate volume of 30 mL and was divided into three aliquots. Urine processing included differential centrifugation and two washes to enrich the bacterial fraction for direct MALDI-TOF MS and direct AST. From a total of 1,638 urine samples, 307 were found to be positive through UF-1000i screening. Among them, 265 had significant growth of a single-microorganism. Direct identification was obtained in 229 (86.42%) out of these 265 samples, and no pathogens were misidentified. Moreover, species-level identification was obtained in 163 (88.59%) out of the 184 samples with Gram-negative bacteria, and 27 (38.03%) out of the 71 samples with Gram-positive bacteria. VITEK 2 AST was performed for 117 samples with a single-microorganism. Enterobacteriaceae data showed an agreement rate of antimicrobial categories of 94.83% (1,229/1,296), with minor, major, and very major error rates of 4.17% (54/1,296), 0.92% (12/1,296), and 0.08% (1/1,296), respectively. For Enterococcus spp., the overall categorical agreement was 92.94% (158/170), with a minor error rate of 2.94% (5/170) and major error rate of 4.12% (7/170). The turnaround time of this combined protocol to diagnose UTIs was 1 h for pathogen identification and 6-24 h for AST; noteworthily, only 6-8 h are needed for AST of Enterobacteriaceae using the VITEK 2 system. Overall, our findings show that the combination of flow cytometry, MALDI-TOF MS, and VITEK 2 provided a direct, rapid, and reliable identification and AST method for assessing urine samples, especially for Gram-negative bacterial infections.
Keywords: MALDI-TOF MS; antimicrobial susceptibility; bacterial identification; rapid diagnosis; urinary tract infections; urine.
Figures
References
-
- Burillo A., Rodríguez-Sánchez B., Ramiro A., Cercenado E., Rodríguez-Créixems M., Bouza E. (2014). Gram-stain plus MALDI-TOF MS (Matrix-assisted laser desorption ionization-time of flight mass spectrometry) for a rapid diagnosis of urinary tract infection. PLoS One 9:e86915. 10.1371/journal.pone.0086915 - DOI - PMC - PubMed
-
- Clinical and Laboratory Standards Institute [CLSI] (2018a). Development of in Vitro Susceptibility Testing Criteria and Quality Control Parameters, 5th Edn. Wayne, PA: CLSI.
-
- Clinical and Laboratory Standards Institute [CLSI] (2018b). Performance Standards for Antimicrobial Susceptibility Testing; 28th Informational Supplement M100-S28. Wayne, PA: CLSI.
LinkOut - more resources
Full Text Sources
Miscellaneous