In vitro Evaluation of Photodynamic Effects Against Biofilms of Dermatophytes Involved in Onychomycosis
- PMID: 31231330
- PMCID: PMC6568038
- DOI: 10.3389/fmicb.2019.01228
In vitro Evaluation of Photodynamic Effects Against Biofilms of Dermatophytes Involved in Onychomycosis
Abstract
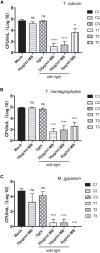
Dermatophytes are the most common cause of onychomycosis, counting for 90% fungal nail infection. Although dermatophyte pathogens are normally susceptible to antifungal agents, onychomycosis often results in refractory chronic disease, and the formation of biofilms frequently underlines the inadequate responses and resistance to standard antifungal treatment. Numerous in vitro and in vivo antimicrobial photodynamic therapy (aPDT) studies have shown biofilm eradication or substantial reduction, however, such investigation has not yet been expanded to the biofilms of dermatophytes involved in onychomycosis. To shed a light on the potential application of aPDT in the clinic management of onychomycosis, in particular with the manifestation of dermatophytoma, we investigated photodynamic effects on the viabilities and the drug susceptibilities of the biofilm of dermatophytes in vitro. Here, methylene blue at the concentration of 8, 16, and 32 μg/ml applied as photosensitizing agent and LED (635 ± 10 nm, 60 J/cm2) as light source were employed against six strains of Trichophyton rubrum, ten strains of Trichophyton mentagrophytes and three strains of Microsporum gypseum isolated from clinical specimens. Our results indicated highly efficient photodynamic inhibition, exhibiting CFU (colony forming unit) reduction up to 4.6 log10, 4.3 log10, and 4.7 log10 against the biofilms formed by T. rubrum, T. mentagrophytes, and M. gypseum, respectively. Subjected biofilms displayed considerable decreases in SMICs (sessile minimum inhibitory concentrations) to multiple antifungal agents when compared with untreated groups, indicating the biofilms of dermatophytes became more susceptible to conventional antifungal drugs after aPDT. Additionally, the obliteration of biofilm after aPDT could be observed as shattered and ruptured structures being evident in SEM (Scanning Electron Microscopy) images. These findings suggest that aPDT is an attractive alternative treatment holding great promise for combating recalcitrant onychomycosis associated with the biofilm formation.
Keywords: aPDT; biofilm; dermatophytes; dermatophytoma; onychomycosis.
Figures



Similar articles
-
Comparative Transcriptome Analysis of T. rubrum, T. mentagrophytes, and M. gypseum Dermatophyte Biofilms in Response to Photodynamic Therapy.Mycopathologia. 2024 Jun 18;189(4):59. doi: 10.1007/s11046-024-00865-y. Mycopathologia. 2024. PMID: 38890181
-
Evaluation of the Effects of Photodynamic Therapy Alone and Combined with Standard Antifungal Therapy on Planktonic Cells and Biofilms of Fusarium spp. and Exophiala spp.Front Microbiol. 2016 Apr 27;7:617. doi: 10.3389/fmicb.2016.00617. eCollection 2016. Front Microbiol. 2016. PMID: 27199946 Free PMC article.
-
Photodynamic inactivation by hypericin-P123 on azole-resistant isolates of the Trichophyton rubrum complex as planktonic cells and biofilm.Photodiagnosis Photodyn Ther. 2023 Dec;44:103875. doi: 10.1016/j.pdpdt.2023.103875. Epub 2023 Nov 1. Photodiagnosis Photodyn Ther. 2023. PMID: 37923285
-
A review of recent research on antifungal agents against dermatophyte biofilms.Med Mycol. 2021 Apr 6;59(4):313-326. doi: 10.1093/mmy/myaa114. Med Mycol. 2021. PMID: 33418566 Review.
-
Evidence for biofilms in onychomycosis.G Ital Dermatol Venereol. 2019 Feb;154(1):50-55. doi: 10.23736/S0392-0488.18.06001-7. Epub 2018 Apr 19. G Ital Dermatol Venereol. 2019. PMID: 29683287 Review.
Cited by
-
Antimicrobial Photodynamic Therapy for Superficial, Skin, and Mucosal Fungal Infections: An Update.Microorganisms. 2025 Jun 17;13(6):1406. doi: 10.3390/microorganisms13061406. Microorganisms. 2025. PMID: 40572295 Free PMC article. Review.
-
Antifungal and Antibiofilm Activity of Riparin III against Dermatophytes.J Fungi (Basel). 2023 Feb 9;9(2):231. doi: 10.3390/jof9020231. J Fungi (Basel). 2023. PMID: 36836345 Free PMC article.
-
White piedra: fungal extracellular matrix formation and its importance in pathogenesis. An ultrastructural study.An Bras Dermatol. 2025 Jul-Aug;100(4):501140. doi: 10.1016/j.abd.2025.501140. Epub 2025 Jul 3. An Bras Dermatol. 2025. PMID: 40614550 Free PMC article.
-
The photosensitizer-based therapies enhance the repairing of skin wounds.Front Med (Lausanne). 2022 Aug 11;9:915548. doi: 10.3389/fmed.2022.915548. eCollection 2022. Front Med (Lausanne). 2022. PMID: 36035433 Free PMC article. Review.
-
Study on the anti-biofilm mechanism of 1,8-cineole against Fusarium solani species complex.Front Pharmacol. 2022 Oct 14;13:1010593. doi: 10.3389/fphar.2022.1010593. eCollection 2022. Front Pharmacol. 2022. PMID: 36330094 Free PMC article.
References
-
- Al-Ahmad A., Walankiewicz A., Hellwig E., Follo M., Tennert C., Wittmer A., et al. (2016). Photoinactivation using visible light plus water-filtered infrared-A (vis+wIRA) and chlorine e6 (Ce6) eradicates planktonic periodontal pathogens and subgingival biofilms. Front. Microbiol. 7:1900. 10.3389/fmicb.2016.01900 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

