Clinical and Immunological Profile of Anti-factor H Antibody Associated Atypical Hemolytic Uremic Syndrome: A Nationwide Database
- PMID: 31231391
- PMCID: PMC6567923
- DOI: 10.3389/fimmu.2019.01282
Clinical and Immunological Profile of Anti-factor H Antibody Associated Atypical Hemolytic Uremic Syndrome: A Nationwide Database
Abstract
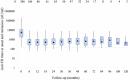
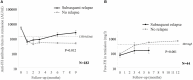
Background: Atypical hemolytic uremic syndrome (aHUS), an important cause of acute kidney injury (AKI), is characterized by dysregulation of the alternative complement pathway. Autoantibodies to factor H (FH), a chief regulator of this pathway, account for a distinct subgroup. While high anti-FH titers predict relapse, they do not correlate well with disease activity and their functional characterization is required. Methods: Of 781 patients <18-year-old of aHUS in the nationwide database from 2007 to 2018, 436 (55.8%) had anti-FH antibodies. Clinical features and outcome of patients managed in the last 6-year (n = 317) were compared to before (n = 119). In plasma samples of 44 patients, levels of serial circulating FH immune complexes (CIC), free FH, soluble terminal complement complex (sC5b-9), sheep red blood cell (SRBC) lysis and epitope specificity (n = 8) were examined. Functional renal reserve, ambulatory hypertension, left ventricular hypertrophy (LVH), and proteinuria were evaluated in a subset. Results: Patients presented with markedly elevated anti-FH titers (10,633.2 ± 998.5 AU/ml). Management varied by center, comprising plasma exchange (PEX; 77.5%) and immunosuppression (73.9%). Patients managed in the last 6-year showed better renal survival at mean 28.5 ± 27.3 months (log rank P = 0.022). Mean anti-FH titers stayed 700-1,164 AU/ml during prolonged follow-up, correlating with CIC. Patients with relapse had lower free-FH during remission [Generalized estimating equations (GEE), P = 0.001]; anti-FH levels ≥1,330 AU/ml and free FH ≤440 mg/l predicted relapse (hazards ratio, HR 6.3; P = 0.018). Epitope specificity was similar during onset, remission and relapse. Antibody titer ≥8,000 AU/ml (HR 2.23; P = 0.024), time to PEX ≥14 days (HR 2.09; P = 0.071) and PEX for <14 days (HR 2.60; P = 0.017) predicted adverse renal outcomes. Combined PEX and immunosuppression improved long-term outcomes (HR 0.37; P = 0.026); maintenance therapy reduced risk of relapses (HR 0.11; P < 0.001). At 4.4±2.5 year, median renal reserve was 15.9%; severe ambulatory, masked and pre-hypertension were found in 38, 30, and 18%, respectively. Proteinuria and LVH occurred in 58 and 28% patients, respectively. Conclusion: Prompt recognition and therapy with PEX and immunosuppression, is associated with satisfactory outcomes. Free-FH predicts early relapses in patients with high anti-FH titers. A significant proportion of impaired functional reserve, ambulatory hypertension, proteinuria and LVH highlight the need for vigilant long-term follow-up.
Keywords: atypical hemolytic uremic syndrome; factor H; plasma exchange; renal reserve; thrombotic microangiopathy.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

