SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes
- PMID: 31231549
- PMCID: PMC6549181
- DOI: 10.1038/s41420-019-0181-7
SARS-Coronavirus Open Reading Frame-8b triggers intracellular stress pathways and activates NLRP3 inflammasomes
Abstract
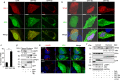
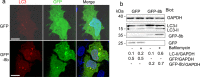
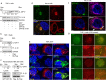
The SARS (severe acute respiratory syndrome) outbreak was caused by a coronavirus (CoV) named the SARS-CoV. SARS pathology is propagated both by direct cytotoxic effects of the virus and aberrant activation of the innate immune response. Here, we identify several mechanisms by which a SARS-CoV open reading frame (ORF) activates intracellular stress pathways and targets the innate immune response. We show that ORF8b forms insoluble intracellular aggregates dependent on a valine at residue 77. Aggregated ORF8b induces endoplasmic reticulum (ER) stress, lysosomal damage, and subsequent activation of the master regulator of the autophagy and lysosome machinery, Transcription factor EB (TFEB). ORF8b causes cell death in epithelial cells, which is partially rescued by reducing its ability to aggregate. In macrophages, ORF8b robustly activates the NLRP3 inflammasome by providing a potent signal 2 required for activation. Mechanistically, ORF8b interacts directly with the Leucine Rich Repeat domain of NLRP3 and localizes with NLRP3 and ASC in cytosolic dot-like structures. ORF8b triggers cell death consistent with pyroptotic cell death in macrophages. While in those cells lacking NLRP3 accumulating ORF8b cytosolic aggregates cause ER stress, mitochondrial dysfunction, and caspase-independent cell death.
Keywords: Cell death and immune response; Inflammasome.
Conflict of interest statement
Conflict of interestThe authors declare that they have no conflict of interest.
Figures



References
-
- Balboni A, Battilani M, Prosperi S. The SARS-like coronaviruses: the role of bats and evolutionary relationships with SARS coronavirus. New Microbiol. 2012;35:1–16. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

