Indoleamine 2,3-Dioxygenase Activity During Acute Toxoplasmosis and the Suppressed T Cell Proliferation in Mice
- PMID: 31231617
- PMCID: PMC6561234
- DOI: 10.3389/fcimb.2019.00184
Indoleamine 2,3-Dioxygenase Activity During Acute Toxoplasmosis and the Suppressed T Cell Proliferation in Mice
Abstract
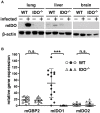
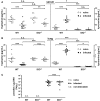
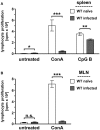
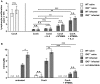
Toxoplasma gondii (T. gondii) is an obligate intracellular parasite and belongs to the phylum Apicomplexa. T. gondii is of medical and veterinary importance, because T. gondii causes the parasitic disease toxoplasmosis. In human cells, the interferon-gamma inducible indoleamine 2,3-dioxygenase 1 (IDO1) is an antimicrobial effector mechanism that degrades tryptophan to kynurenine and thus limits pathogen proliferation in vitro. Furthermore, IDO is described to have immunosuppressive properties, e.g., regulatory T cell differentiation and T cell suppression in humans and mice. However, there is only little known about the role of IDO1 in mice during acute toxoplasmosis. To shed further light on the role of mIDO1 in vivo, we have used a specifically adjusted experimental model. Therein, we infected mIDO1-deficient (IDO-/-) C57BL/6 mice and appropriate wild-type (WT) control mice with a high dose of T. gondii ME49 tachyozoites (type II strain) via the intraperitoneal route and compared the phenotype of IDO-/- and WT mice during acute toxoplasmosis. During murine T. gondii infection, we found mIDO1 mRNA and mIDO1 protein, as well as mIDO1-mediated tryptophan degradation in lungs of WT mice. IDO-/- mice show no tryptophan degradation in the lung during infection. Even though T. gondii is tryptophan auxotroph and rapidly replicates during acute infection, the parasite load was similar in IDO-/- mice compared to WT mice 7 days post-infection. IDO1 is described to have immunosuppressive properties, and since T cell suppression is observed during acute toxoplasmosis, we analyzed the possible involvement of mIDO1. Here, we did not find differences in the intensity of ex vivo mitogen stimulated T cell proliferation between WT and IDO-/- mice. Concomitant nitric oxide synthase inhibition and interleukin-2 supplementation increased the T cell proliferation from both genotypes drastically, but not completely. In sum, we analyzed the involvement of mIDO1 during acute murine toxoplasmosis in our specifically adjusted experimental model and found a definite mIDO1 induction. Nevertheless, mIDO1 seems to be functional redundant as an antiparasitic defense mechanism during acute toxoplasmosis in mice. Furthermore, we suggest that the systemic T cell suppression observed during acute toxoplasmosis is influenced by nitric oxide activity and IL-2 deprivation.
Keywords: IDO; T cell suppression; Toxoplasma gondii; kynurenine; mouse.
Figures
Similar articles
-
L-tryptophan-L-kynurenine pathway metabolism accelerated by Toxoplasma gondii infection is abolished in gamma interferon-gene-deficient mice: cross-regulation between inducible nitric oxide synthase and indoleamine-2,3-dioxygenase.Infect Immun. 2002 Feb;70(2):779-86. doi: 10.1128/IAI.70.2.779-786.2002. Infect Immun. 2002. PMID: 11796611 Free PMC article.
-
The mechanism of interferon-gamma induced anti Toxoplasma gondii by indoleamine 2,3-dioxygenase and/or inducible nitric oxide synthase vary among tissues.Adv Exp Med Biol. 2003;527:97-103. doi: 10.1007/978-1-4615-0135-0_11. Adv Exp Med Biol. 2003. PMID: 15206721
-
Inhibition of increased indoleamine 2,3-dioxygenase activity attenuates Toxoplasma gondii replication in the lung during acute infection.Cytokine. 2012 Aug;59(2):245-51. doi: 10.1016/j.cyto.2012.04.022. Epub 2012 May 18. Cytokine. 2012. PMID: 22609210
-
Tryptophan Metabolism in Obesity: The Indoleamine 2,3-Dioxygenase-1 Activity and Therapeutic Options.Adv Exp Med Biol. 2024;1460:629-655. doi: 10.1007/978-3-031-63657-8_21. Adv Exp Med Biol. 2024. PMID: 39287867 Review.
-
Investigating the Role of Indoleamine 2,3-Dioxygenase in Acute Myeloid Leukemia: A Systematic Review.Front Immunol. 2021 Mar 10;12:651687. doi: 10.3389/fimmu.2021.651687. eCollection 2021. Front Immunol. 2021. PMID: 33777052 Free PMC article.
Cited by
-
The pathogenicity and virulence of Toxoplasma gondii.Virulence. 2021 Dec;12(1):3095-3114. doi: 10.1080/21505594.2021.2012346. Virulence. 2021. PMID: 34895084 Free PMC article.
-
Deficiency in indoleamine-2, 3-dioxygenase induces upregulation of guanylate binding protein 1 and inducible nitric oxide synthase expression in the brain during cerebral infection with Toxoplasma gondii in genetically resistant BALB/c mice but not in genetically susceptible C57BL/6 mice.Microbes Infect. 2022 Apr-May;24(3):104908. doi: 10.1016/j.micinf.2021.104908. Epub 2021 Nov 13. Microbes Infect. 2022. PMID: 34781010 Free PMC article.
-
Metabolic Regulation of Immune Responses to Mycobacterium tuberculosis: A Spotlight on L-Arginine and L-Tryptophan Metabolism.Front Immunol. 2021 Feb 9;11:628432. doi: 10.3389/fimmu.2020.628432. eCollection 2020. Front Immunol. 2021. PMID: 33633745 Free PMC article. Review.
-
Lymphotoxin β Receptor: a Crucial Role in Innate and Adaptive Immune Responses against Toxoplasma gondii.Infect Immun. 2021 May 17;89(6):e00026-21. doi: 10.1128/IAI.00026-21. Print 2021 May 17. Infect Immun. 2021. PMID: 33753412 Free PMC article.
-
Group B streptococci infection model shows decreased regulatory capacity of cord blood cells.Pediatr Res. 2022 Nov;92(5):1407-1416. doi: 10.1038/s41390-021-01880-1. Epub 2022 Feb 14. Pediatr Res. 2022. PMID: 35165359 Free PMC article.
References
-
- Adams L. B., Hibbs J. B., Taintor R. R., Krahenbuhl J. L. (1990). Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J. Immunol. 144, 2725–2729. - PubMed
-
- Albina J. E., Abate J. A., Henry W. L., Jr. (1991). Nitric oxide production is required for murine resident peritoneal macrophages to suppress mitogen-stimulated T cell proliferation. Role of IFN-gamma in the induction of the nitric oxide-synthesizing pathway. J. Immunol. 147, 144–148. - PubMed
-
- Araújo E. F., Loures F. V., Bazan S. B., Feriotti C., Pina A., Schanoski A. S., et al. . (2014). Indoleamine 2,3-dioxygenase controls fungal loads and immunity in paracoccidioidomicosis but is more important to susceptible than resistant hosts. PLoS Negl. Trop. Dis. 8:e3330. 10.1371/journal.pntd.0003330 - DOI - PMC - PubMed
-
- Bando H., Lee Y., Sakaguchi N., Pradipta A., Ma J. S., Tanaka S., et al. . (2018). Inducible nitric oxide synthase is a key host factor for toxoplasma GRA15-dependent disruption of the gamma interferon-induced antiparasitic human response. MBio 9:e01738-18. 10.1128/mBio.01738-18 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

