Transcriptome Analysis of Small Molecule-Mediated Astrocyte-to-Neuron Reprogramming
- PMID: 31231645
- PMCID: PMC6558402
- DOI: 10.3389/fcell.2019.00082
Transcriptome Analysis of Small Molecule-Mediated Astrocyte-to-Neuron Reprogramming
Abstract
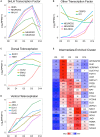
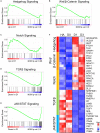
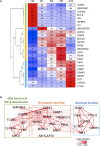
Chemical reprogramming of astrocytes into neurons represents a promising approach to regenerate new neurons for brain repair, but the underlying mechanisms driving this trans-differentiation process are not well understood. We have recently identified four small molecules - CHIR99021, DAPT, LDN193189, and SB431542 - that can efficiently reprogram cultured human fetal astrocytes into functional neurons. Here we employ the next generation of RNA-sequencing technology to investigate the transcriptome changes during the astrocyte-to-neuron (AtN) conversion process. We found that the four small molecules can rapidly activate the hedgehog signaling pathway while downregulating many glial genes such as FN1 and MYL9 within 24 h of treatment. Chemical reprogramming is mediated by several waves of differential gene expression, including upregulation of hedgehog, Wnt/β-catenin, and Notch signaling pathways, together with downregulation of TGF-β and JAK/STAT signaling pathways. Our gene network analyses reveal many well-connected hub genes such as repulsive guidance molecule A (RGMA), neuronatin (NNAT), neurogenin 2 (NEUROG2), NPTX2, MOXD1, JAG1, and GAP43, which may coordinate the chemical reprogramming process. Together, these findings provide critical insights into the molecular cascades triggered by a combination of small molecules that eventually leads to chemical conversion of astrocytes into neurons.
Keywords: astrocyte; chemical reprogramming; neuron; signaling pathway; transcriptome.
Figures
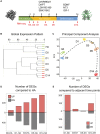
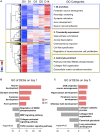
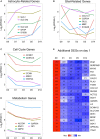
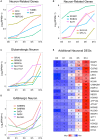
Similar articles
-
Transcriptomic analyses of NeuroD1-mediated astrocyte-to-neuron conversion.Dev Neurobiol. 2022 Jul;82(5):375-391. doi: 10.1002/dneu.22882. Epub 2022 May 23. Dev Neurobiol. 2022. PMID: 35606902 Free PMC article.
-
Generation of a Pure Culture of Neuron-like Cells with a Glutamatergic Phenotype from Mouse Astrocytes.Biomedicines. 2022 Apr 18;10(4):928. doi: 10.3390/biomedicines10040928. Biomedicines. 2022. PMID: 35453678 Free PMC article.
-
Chemical Conversion of Human Fetal Astrocytes into Neurons through Modulation of Multiple Signaling Pathways.Stem Cell Reports. 2019 Mar 5;12(3):488-501. doi: 10.1016/j.stemcr.2019.01.003. Epub 2019 Feb 7. Stem Cell Reports. 2019. PMID: 30745031 Free PMC article.
-
In situ chemical reprogramming of astrocytes into neurons: A new hope for the treatment of central neurodegenerative diseases?Eur J Pharmacol. 2024 Nov 5;982:176930. doi: 10.1016/j.ejphar.2024.176930. Epub 2024 Aug 22. Eur J Pharmacol. 2024. PMID: 39179093 Review.
-
Glial-Neuron Transformation by "Chemical Cocktail".ACS Chem Neurosci. 2019 Jan 16;10(1):42-43. doi: 10.1021/acschemneuro.8b00684. Epub 2018 Dec 20. ACS Chem Neurosci. 2019. PMID: 30571085 Review.
Cited by
-
Cell Reprogramming for Regeneration and Repair of the Nervous System.Biomedicines. 2022 Oct 17;10(10):2598. doi: 10.3390/biomedicines10102598. Biomedicines. 2022. PMID: 36289861 Free PMC article. Review.
-
Efficient and rapid conversion of human astrocytes and ALS mouse model spinal cord astrocytes into motor neuron-like cells by defined small molecules.Mil Med Res. 2020 Sep 6;7(1):42. doi: 10.1186/s40779-020-00271-7. Mil Med Res. 2020. PMID: 32892745 Free PMC article.
-
A Hybrid Machine Learning and Network Analysis Approach Reveals Two Parkinson's Disease Subtypes from 115 RNA-Seq Post-Mortem Brain Samples.Int J Mol Sci. 2022 Feb 25;23(5):2557. doi: 10.3390/ijms23052557. Int J Mol Sci. 2022. PMID: 35269707 Free PMC article.
-
Transcriptomic analyses of NeuroD1-mediated astrocyte-to-neuron conversion.Dev Neurobiol. 2022 Jul;82(5):375-391. doi: 10.1002/dneu.22882. Epub 2022 May 23. Dev Neurobiol. 2022. PMID: 35606902 Free PMC article.
-
Reprogramming Glial Cells into Functional Neurons for Neuro-regeneration: Challenges and Promise.Neurosci Bull. 2021 Nov;37(11):1625-1636. doi: 10.1007/s12264-021-00751-3. Epub 2021 Jul 20. Neurosci Bull. 2021. PMID: 34283396 Free PMC article. Review.
References
-
- Borghese L., Dolezalova D., Opitz T., Haupt S., Leinhaas A., Steinfarz B., et al. (2010). Inhibition of Notch signaling in human embryonic stem cell–derived neural stem cells delays G1/S phase transition and accelerates neuronal differentiation in vitro and in vivo. Stem Cells 28 955–964. 10.1002/stem.408 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

