Roles for Golgi Glycans in Oogenesis and Spermatogenesis
- PMID: 31231650
- PMCID: PMC6566014
- DOI: 10.3389/fcell.2019.00098
Roles for Golgi Glycans in Oogenesis and Spermatogenesis
Abstract
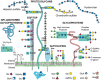
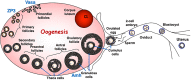
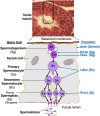
Glycosylation of proteins by N- and O-glycans or glycosaminoglycans (GAGs) mostly begins in the endoplasmic reticulum and is further orchestrated in the Golgi compartment via the action of >100 glycosyltransferases that reside in this complex organelle. The synthesis of glycolipids occurs in the Golgi, also by resident glycosyltransferases. A defect in the glycosylation machinery may impair the functions of glycoproteins and other glycosylated molecules, and lead to a congenital disorder of glycosylation (CDG). Spermatogenesis in the male and oogenesis in the female are tightly regulated differentiation events leading to the production of functional gametes. Insights into roles for glycans in gamete production have been obtained from mutant mice following deletion or inactivation of genes that encode a glycosylation activity. In this review, we will summarize the effects of altering the synthesis of N-glycans, O-glycans, proteoglycans, glycophosphatidylinositol (GPI) anchored proteins, and glycolipids during gametogenesis in the mouse. Glycosylation genes whose deletion causes embryonic lethality have been investigated following conditional deletion using various Cre recombinase transgenes with a cell-type specific promoter. The potential effects of mutations in corresponding glycosylation genes of humans will be discussed in relation to consequences to fertility and potential for use in contraception.
Keywords: Golgi; fertility; glycans; glycosylation; oogenesis; spermatogenesis.
Figures
Similar articles
-
What Have We Learned from Glycosyltransferase Knockouts in Mice?J Mol Biol. 2016 Aug 14;428(16):3166-3182. doi: 10.1016/j.jmb.2016.03.025. Epub 2016 Mar 31. J Mol Biol. 2016. PMID: 27040397 Free PMC article. Review.
-
Modeling Congenital Disorders of N-Linked Glycoprotein Glycosylation in Drosophila melanogaster.Front Genet. 2018 Oct 2;9:436. doi: 10.3389/fgene.2018.00436. eCollection 2018. Front Genet. 2018. PMID: 30333856 Free PMC article. Review.
-
[Congenital disorders of glycosylation].Ann Pharm Fr. 2003;61(5):330-9. Ann Pharm Fr. 2003. PMID: 13130291 Review. French.
-
How Golgi glycosylation meets and needs trafficking: the case of the COG complex.Glycobiology. 2011 Jul;21(7):853-63. doi: 10.1093/glycob/cwq179. Epub 2010 Nov 26. Glycobiology. 2011. PMID: 21112967 Review.
-
Purification, biosynthesis and cellular localization of a major 125-kDa glycophosphatidylinositol-anchored membrane glycoprotein of Saccharomyces cerevisiae.Eur J Biochem. 1991 Jan 30;195(2):439-48. doi: 10.1111/j.1432-1033.1991.tb15723.x. Eur J Biochem. 1991. PMID: 1847682
Cited by
-
Deletion of Mgat2 in spermatogonia blocks spermatogenesis.Front Cell Dev Biol. 2024 Sep 19;12:1428715. doi: 10.3389/fcell.2024.1428715. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39364139 Free PMC article.
-
The synergic impact of decellularized testis scaffold and extracellular vesicles derived from human semen on spermatogonial stem cell survival and differentiation.Biomed Eng Online. 2025 Jul 26;24(1):94. doi: 10.1186/s12938-025-01424-2. Biomed Eng Online. 2025. PMID: 40713602 Free PMC article.
-
Proteoglycans in the Pathogenesis of Hormone-Dependent Cancers: Mediators and Effectors.Cancers (Basel). 2020 Aug 24;12(9):2401. doi: 10.3390/cancers12092401. Cancers (Basel). 2020. PMID: 32847060 Free PMC article. Review.
-
Alteration of the metabolite interconversion enzyme in sperm and Sertoli cell of non-obstructive azoospermia: a microarray data and in-silico analysis.Sci Rep. 2024 Oct 29;14(1):25965. doi: 10.1038/s41598-024-77875-9. Sci Rep. 2024. PMID: 39472682 Free PMC article.
-
Gene transcriptional profiles in gonads of Bacillus taxa (Phasmida) with different cytological mechanisms of automictic parthenogenesis.Zoological Lett. 2022 Nov 26;8(1):14. doi: 10.1186/s40851-022-00197-z. Zoological Lett. 2022. PMID: 36435814 Free PMC article.
References
-
- Akama T. O., Nakagawa H., Wong N. K., Sutton-Smith M., Dell A., Morris H. R., et al. (2006). Essential and mutually compensatory roles of {alpha}-mannosidase II and {alpha}-mannosidase IIx in N-glycan processing in vivo in mice. Proc. Natl. Acad. Sci. U.S.A. 103 8983–8988. 10.1073/pnas.0603248103 - DOI - PMC - PubMed
-
- Asano M., Furukawa K., Kido M., Matsumoto S., Umesaki Y., Kochibe N., et al. (1997). Growth retardation and early death of beta-1,4-galactosyltransferase knockout mice with augmented proliferation and abnormal differentiation of epithelial cells. EMBO J. 16 1850–1857. 10.1093/emboj/16.8.1850 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

