Developmental exposures to bisphenol S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: a pilot study
- PMID: 31231671
- PMCID: PMC6588183
Developmental exposures to bisphenol S, a BPA replacement, alter estrogen-responsiveness of the female reproductive tract: a pilot study
Abstract
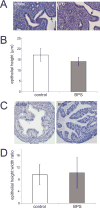
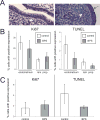
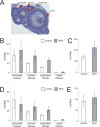
Developmental exposures to bisphenol A (BPA), an estrogen receptor agonist, can disrupt development of the female reproductive tract in rodents and non-human primates. Due to an increased public knowledge of negative health effects associated with BPA exposure, BPA has begun to be phased out of many consumer products and in some cases it has been replaced with structurally similar compounds including bisphenol S (BPS). This study examined CD-1 mice exposed to a low dose of BPS during early development (200 µg/kg/day from gestational day 8 until postnatal day 19). BPS altered expression of estrogen-responsive genes in both the uterus and ovary, and induced increases in ovarian follicular development in pre-pubertal females evaluated at postnatal day 22. Prior studies have revealed that developmental exposures to environmental chemicals including BPA alter the response of animals to hormonal or carcinogen challenges experienced later in life. To evaluate whether early life exposures to BPS alter responses of females to an estrogen challenge, additional females were exposed to ethinyl estradiol from postnatal day 19 through postnatal day 21. BPS-treated females responded abnormally to this estrogen challenge, displaying heightened responses in the uterus and diminished responses in the ovary. Although additional studies are needed to characterize the mechanisms by which BPS alters the female reproductive tract, this pilot study provides evidence that a common BPA replacement chemical may have endocrine disrupting properties.
Keywords: apoptosis; endocrine disruptor; estrogen receptor; ethinyl estradiol; ovarian follicles; proliferation; puberty; uterine endometrium.
Figures





References
-
- Mandrup KR, Jacobsen PR, Isling LK, et al. Effects of perinatal ethinyl estradiol exposure in male and female Wistar rats. Reprod Toxicol. 2013;42:180–91. - PubMed
-
- Soto AM, Rubin BS, Sonnenschein C. Endocrine disruption and the female. In: Gore A, editor. Endocrine-Disrupting Chemicals. Totowa, N.J.: Humana Press; 2007.
-
- McLachlan JA. Commentary: prenatal exposure to diethylstilbestrol (DES): a continuing story. Int J Epidemiol. 2006;35(4):868–70. - PubMed
-
- Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. New England Journal of Medicine. 1971;284(15):878–81. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
