Cardiac interstitial tetraploid cells can escape replicative senescence in rodents but not large mammals
- PMID: 31231694
- PMCID: PMC6565746
- DOI: 10.1038/s42003-019-0453-z
Cardiac interstitial tetraploid cells can escape replicative senescence in rodents but not large mammals
Abstract
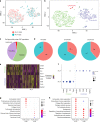
Cardiomyocyte ploidy has been described but remains obscure in cardiac interstitial cells. Ploidy of c-kit+ cardiac interstitial cells was assessed using confocal, karyotypic, and flow cytometric technique. Notable differences were found between rodent (rat, mouse) c-kit+ cardiac interstitial cells possessing mononuclear tetraploid (4n) content, compared to large mammals (human, swine) with mononuclear diploid (2n) content. In-situ analysis, confirmed with fresh isolates, revealed diploid content in human c-kit+ cardiac interstitial cells and a mixture of diploid and tetraploid content in mouse. Downregulation of the p53 signaling pathway provides evidence why rodent, but not human, c-kit+ cardiac interstitial cells escape replicative senescence. Single cell transcriptional profiling reveals distinctions between diploid versus tetraploid populations in mouse c-kit+ cardiac interstitial cells, alluding to functional divergences. Collectively, these data reveal notable species-specific biological differences in c-kit+ cardiac interstitial cells, which could account for challenges in extrapolation of myocardial from preclinical studies to clinical trials.
Keywords: Heart stem cells.
Conflict of interest statement
Competing interestsThe authors declare no competing interests.
Figures






References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

