Non-Invasive Ultrasound Quantification of Scar Tissue Volume Identifies Early Functional Changes During Tendon Healing
- PMID: 31231903
- PMCID: PMC6816309
- DOI: 10.1002/jor.24397
Non-Invasive Ultrasound Quantification of Scar Tissue Volume Identifies Early Functional Changes During Tendon Healing
Abstract
Tendon injuries are very common and disrupt the transmission of forces from muscle to bone, leading to impaired function and quality of life. Successful restoration of tendon function after injury is a challenging clinical problem due to the pathological, scar-mediated manner in which the tendons heal. Currently, there are no standard treatments to modulate scar tissue formation and improve tendon healing. A major limitation to the identification of therapeutic candidates has been the reliance on terminal endpoint metrics of healing in pre-clinical studies, which require a large number of animals and result in destruction of the tissue. To address this limitation, we have identified quantification of scar tissue volume (STV) from ultrasound (US) imaging as a longitudinal, non-invasive metric of tendon healing. STV was strongly correlated with established endpoint metrics of gliding function including gliding resistance and metatarsophalangeal (MTP) flexion angle. However, no associations were observed between STV and structural or material properties. To define the sensitivity of STV to identify differences between functionally discrete tendon healing phenotypes, we utilized S100a4 haploinsufficient mice (S100a4GFP/+ ), which heal with improved gliding function relative to wild-type (WT) littermates. A significant decrease in STV was observed in S100a4GFP/+ repairs, relative to WT at day 14. Taken together, these data suggest US quantification of STV as a means to facilitate the rapid screening of biological and pharmacological interventions to improve tendon healing, and identify promising therapeutic targets, in an efficient, cost-effective manner. © 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc. J Orthop Res 37:2476-2485, 2019.
Keywords: mouse model; range of motion; scar tissue; tendon healing; ultrasound.
© 2019 Orthopaedic Research Society. Published by Wiley Periodicals, Inc.
Figures


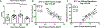
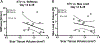

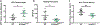
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

