The role of oligodendrocyte gap junctions in neuroinflammation
- PMID: 31232168
- PMCID: PMC6602578
- DOI: 10.1080/19336950.2019.1631107
The role of oligodendrocyte gap junctions in neuroinflammation
Abstract
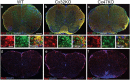
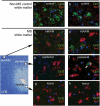
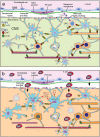
Gap junctions (GJs) provide channels for direct cell-to-cell connectivity serving the homeostasis in several organs of vertebrates including the central (CNS) and peripheral (PNS) nervous systems. GJs are composed of connexins (Cx), which show a highly distinct cellular and subcellular expression pattern. Oligodendrocytes, the myelinating cells of the CNS, are characterized by extensive GJ connectivity with each other as well as with astrocytes. The main oligodendrocyte connexins forming these GJ channels are Cx47 and Cx32. The importance of these channels has been highlighted by the discovery of human diseases caused by mutations in oligodendrocyte connexins, manifesting with leukodystrophy or transient encephalopathy. Experimental models have provided further evidence that oligodendrocyte GJs are essential for CNS myelination and homeostasis, while a strong inflammatory component has been recognized in the absence of oligodendrocyte connexins. Further studies revealed that connexins are also disrupted in multiple sclerosis (MS) brain, and in experimental models of induced inflammatory demyelination. Moreover, induced demyelination was more severe and associated with higher degree of CNS inflammation in models with oligodendrocyte GJ deficiency, suggesting that disrupted connexin expression in oligodendrocytes is not only a consequence but can also drive a pro-inflammatory environment in acquired demyelinating disorders such as MS. In this review, we summarize the current insights from human disorders as well as from genetic and acquired models of demyelination related to oligodendrocyte connexins, with the remaining challenges and perspectives.
Keywords: Gap junction; connexin; myelin; neuroinflammation; oligodendrocytes.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
