Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review
- PMID: 31233229
- PMCID: PMC6590101
- DOI: 10.1002/14651858.CD012065.pub3
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review
Abstract
Background: This is an updated version of the original Cochrane Review published in Issue 12, 2016. This review is one in a series of Cochrane Reviews investigating pair-wise monotherapy comparisons.Epilepsy is a common neurological condition in which abnormal electrical discharges from the brain cause recurrent unprovoked seizures. It is believed that with effective drug treatment, up to 70% of individuals with active epilepsy have the potential to become seizure-free and go into long-term remission shortly after starting drug therapy, the majority of which may be able to achieve remission with a single antiepileptic drug (AED).The correct choice of first-line AED for individuals with newly diagnosed seizures is of great importance and should be based on the highest-quality evidence available regarding the potential benefits and harms of various treatments for an individual.Topiramate and carbamazepine are commonly used AEDs. Performing a synthesis of the evidence from existing trials will increase the precision of results of outcomes relating to efficacy and tolerability, and may help inform a choice between the two drugs.
Objectives: To review the time to treatment failure, remission and first seizure with topiramate compared with carbamazepine when used as monotherapy in people with focal onset seizures (simple or complex focal and secondarily generalised), or generalised onset tonic-clonic seizures (with or without other generalised seizure types).
Search methods: For the latest update we searched the Cochrane Register of Studies (CRS Web), which includes the Cochrane Epilepsy Group Specialized Register and the Cochrane Central Register of Controlled Trials (CENTRAL); MEDLINE (Ovid); ClinicalTrials.gov; and the WHO International Clinical Trials Registry Platform (ICTRP) to 22 May 2018. We imposed no language restrictions. We also contacted pharmaceutical companies and trial investigators.
Selection criteria: Randomised controlled trials (RCTs) comparing monotherapy with either topiramate or carbamazepine in children or adults with focal onset seizures or generalised onset tonic-clonic seizures (with or without other generalised seizure types).
Data collection and analysis: This was an individual participant data (IPD), review. Our primary outcome was time to treatment failure. Our secondary outcomes were time to first seizure post-randomisation, time to six-month remission, time to 12-month remission, and incidence of adverse events. We used Cox proportional hazards regression models to obtain trial-specific estimates of hazard ratios (HRs), with 95% confidence intervals (CIs), using the generic inverse variance method to obtain the overall pooled HR and 95% CI.
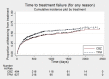
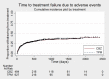
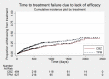
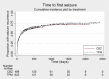
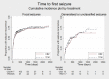
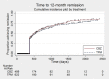
Main results: IPD were available for 1151 of 1239 eligible individuals from two of three eligible studies (93% of the potential data). A small proportion of individuals recruited into these trials had 'unclassified seizures;' for analysis purposes, these individuals are grouped with those with generalised onset seizures. For remission outcomes, a HR < 1 indicated an advantage for carbamazepine, and for first seizure and treatment failure outcomes, a HR < 1 indicated an advantage for topiramate.The main overall results for the primary outcome, time to treatment failure, given as pooled HR adjusted for seizure type were: time to failure for any reason related to treatment 1.16 (95% CI 0.97 to 1.38); time to failure due to adverse events 1.02 (95% CI 0.82 to 1.27); and time to failure due to lack of efficacy 1.46 (95% CI 1.08 to 1.98). Overall results for secondary outcomes were time to first seizure 1.11 (95% CI 0.96 to 1.29); and time to six-month remission 0.88 (0.76 to 1.01). There were no statistically significant differences between the drugs. A statistically significant advantage for carbamazepine was shown for time to 12-month remission: 0.84 (95% CI 0.71 to 0.99).The results of this review are applicable mainly to individuals with focal onset seizures; 81% of individuals included within the analysis experienced seizures of this type at baseline. For individuals with focal onset seizures, a statistically significant advantage for carbamazepine was shown for time to failure for any reason related to treatment (HR 1.21, 95% CI 1.01 to 1.46), time to treatment failure due to lack of efficacy (HR 1.47, 95% CI 1.07 to 2.02), and time to 12-month remission (HR 0.82, 95% CI 0.69 to 0.99). There was no statistically significant difference between topiramate and carbamazepine for 'time to first seizure' and 'time to six-month remission'.Evidence for individuals with generalised tonic-clonic seizures (9% of participants contributing to the analysis), and unclassified seizure types (10% of participants contributing to the analysis) was very limited; no statistically significant differences were found but CIs were wide; therefore we cannot exclude an advantage to either drug, or a difference between drugs.The most commonly reported adverse events with both drugs were drowsiness or fatigue, "pins and needles" (tingling sensation), headache, gastrointestinal disturbance and anxiety or depression. The rate of adverse events was similar across the two drugs.We judged the methodological quality of the included trials generally to be good; however, there was some evidence that the open-label design of the larger of the two trials may have influenced the treatment failure rate within the trial. Hence, we judged the certainty of the evidence for treatment failure to be moderate for individuals with focal onset seizures and low for individuals with generalised onset seizures. For efficacy outcomes (first seizure, remission), we judged the certainty of evidence from this review to be high for individuals with focal onset seizures and moderate for individuals with generalised onset or unclassified seizures.
Authors' conclusions: For individuals with focal onset seizures, there is moderate-certainty evidence that carbamazepine is less likely to be withdrawn and high-certainty evidence that 12-month remission will be achieved earlier than with topiramate. We did not find any differences between the drugs in terms of the other outcomes measured in the review and for individuals with generalised tonic-clonic seizures or unclassified epilepsy; however, we encourage caution in the interpretation of results including small numbers of participants with these seizure types.Future trials should be designed to the highest quality possible and take into consideration masking, choice of population, classification of seizure type, duration of follow-up, choice of outcomes and analysis, and presentation of results.
Conflict of interest statement
SJN: none known MS: none known CTS: none known AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Tony Marson is part funded by National Institute for Health Research Collaboration for Leadership in Applied Health Research and Care North West Coast (NIHR CLAHRC NWC).
Figures

















Update of
-
Topiramate versus carbamazepine monotherapy for epilepsy: an individual participant data review.Cochrane Database Syst Rev. 2016 Dec 6;12(12):CD012065. doi: 10.1002/14651858.CD012065.pub2. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2019 Jun 24;6:CD012065. doi: 10.1002/14651858.CD012065.pub3. PMID: 27922722 Free PMC article. Updated.
References
References to studies included in this review
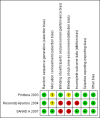
Privitera 2003 {published data only}
-
- Ben‐Menachem E, Privitera MD, Neto W, Wu SC. Response to topiramate (TPM), carbamazepine (CBZ) or valproate (VPA) by seizure type in newly diagnosed epilepsy. Epilepsia 2005;46(Suppl 6):274‐5, Abstract no: p843.
-
- Edwards JC, Privitera MD, Neto W, Wu S. Topiramate (TPM) vs carbamazepine (CBZ) and valproate (VPA) in newly diagnosed epilepsy: effectiveness according to seizure type. Epilepsia 2004;45(Suppl 7):139‐40, Abstract no: 1.369.
-
- Privitera MD, Arroyo S, Squires L, Wang S, Twyman R. 100 mg/day topiramate as the initial target dose in newly diagnosed epilepsy. Epilepsia 2003;44(Suppl 9):270, Abstract no: 2.5.
-
- Privitera MD, Brodie MJ, Mattson RH, Chadwick DW, Neto W, Wang S. Topiramate, carbamazepine and valproate monotherapy: double‐blind comparison in newly diagnosed epilepsy. Acta Neurologica Scandinavica 2003;107(3):165‐75. - PubMed
-
- Privitera MD, Brodie MJ, Neto W, Wang S. Topiramate vs. investigator choice of carbamazepine or valproate as monotherapy in newly diagnosed epilepsy. Epilepsia 2000;41(Suppl 7):93‐4, Abstract no: 2.019.
Resendiz‐Aparicio 2004 {published data only}
-
- Resendiz‐Aparicio JC, Rodriguez‐Rodriguez E, Contreras‐Bernal J, Ceja‐Moreno H, Barragan‐Perez E, Garza‐Morales S, et al. A randomised open trial comparing monotherapy with topiramate versus carbamazepine in the treatment of paediatric patients with recently diagnosed epilepsy. Revista de Neurologia 2004;39(3):201‐4. - PubMed
SANAD A 2007 {published data only}
-
- Marson A, Smith D, Tudur Smith C, Williamson P, Jacoby A, Chadwick D. Carbamazepine versus gabapentin, lamotrigine, oxcarbazepine and topiramate for epilepsy: results from arm A of the SANAD trial. Epilepsia 2006;47(Suppl 4):272.
-
- Marson AG, Al‐Kharusi AM, Alwaidh M, Appleton R, Baker GA, Chadwick DW, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet 2007;369(9566):1000‐15. - PMC - PubMed
-
- Marson AG, Appleton R, Baker GA, Chadwick DW, Doughty J, Eaton B, et al. A randomised controlled trial examining the longer‐term outcomes of standard versus new antiepileptic drugs. The SANAD trial. Health Technology Assessment 2007;11(37):iii‐iv, ix‐x, 1‐134. - PubMed
References to studies excluded from this review
Kang 2007 {published data only}
-
- Kang HC, Eun BL, Lee CW, Moon HK, Kim JS, Kim DW, et al. A multicenter, randomized, open‐labeled, clinical study to evaluate the effect on cognitive and behavioral function of topiramate compared with carbamazepine as monotherapy in children with benign rolandic epilepsy. Epilepsia 2006;47(Suppl 4):138, Abstract no: 2.057.
-
- Kang HC, Eun BL, Wu Lee C, Ku Moon H, Kim JS, Wook Kim D, et al. The effects on cognitive function and behavioural problems of topiramate compared to carbamazepine as monotherapy for children with benign rolandic epilepsy. Epilepsia 2007;48(9):1716‐23. - PubMed
-
- Lim K, Kim HD, Korean BRE Study Group. Low‐dose topiramate compared with carbamazepine in treating benign rolandic epilepsy. Epilepsia 2004;45(Suppl 7):322‐3, Abstract no: 2.390.
Additional references
Annegers 1999
-
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia 1999;40(4):502‐6. - PubMed
Bell 2014
-
- Bell GS, Neligan A, Sander JW. An unknown quantity—the worldwide prevalence of epilepsy. Epilepsia 2014;55(7):958‐62. - PubMed
Brodie 1996
-
- Brodie MJ, Dichter MA. Antiepileptic drugs. New England Journal of Medicine 1996;334(3):168‐75. - PubMed
Bromley 2014
Cockerell 1995
-
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8968):140‐4. - PubMed
Coulter 1993
-
- Coulter DA, Sombati S, DeLorenzo RJ. Selective effects of topiramate on sustained repetitive firing and spontaneous bursting in cultured hippocampal neurons. Epilepsia 1993;34(Suppl 2):123. - PubMed
Excel 2010 [Computer program]
-
- Microsoft. Microsoft Excel. Version accessed 13 May 2016. Redmond (WA): Microsoft, 2010.
French 2007
-
- French JA, Kanner AM, Bautista J, Abou‐Khalil B, Browne T, Harden CL, et al. Appendix C: Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new onset epilepsy: Report of the Therapeutics and Technology Assessment Subcommittee and Quality Standards Subcommittee of the American Academy of Neurology and the American Epilepsy Society. CONTINUUM Lifelong Learning in Neurology 2007;13(4):203‐11. - PubMed
Gilliam 2003
-
- Gilliam FG, Veloso F, Bomhof MA, Gazda SK, Biton V, Ter Bruggen JP, et al. A dose‐comparison trial of topiramate as monotherapy in recently diagnosed partial epilepsy. Neurology 2003;60(2):196‐202. - PubMed
Global Burden of Disease Study 2013
-
- Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(9995):743‐800. - PMC - PubMed
GRADEPro GDT 2015 [Computer program]
-
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 13 May 2016. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Hauser 1993
-
- Hauser WA, Annegers JF, Kurland LT. Incidence of epilepsy and unprovoked seizures in Rochester, Minnesota 1935‐1984. Epilepsia 1993;34(3):453‐68. - PubMed
Higgins 2003
Higgins 2017
-
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. Cochrane.
Hirtz 2007
-
- Hirtz D, Thurman DJ, Gwinn‐Hardy K, Mohamed M, Chaudhuri AR, Zalutsky R. How common are the "common" neurologic disorders?. Neurology 2007;68(5):326‐37. - PubMed
Hultcrantz 2017
Hunt 2008
-
- Hunt S, Russell A, Smithson WH, Parsons L, Robertson I, Waddell R, et al. Topiramate in pregnancy: preliminary experience from the UK Epilepsy and Pregnancy Register. Neurology 2008;71(4):272‐6. - PubMed
ILAE 1998
-
- ILAE Commission on Antiepileptic Drugs. Considerations on designing clinical trials to evaluate the place of new antiepileptic drugs in the treatment of newly diagnosed and chronic patients with epilepsy. Epilepsia 1998;39(7):799‐803. - PubMed
ILAE 2006
-
- Glauser T, Ben‐Menachem E, Bourgeois B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE treatment guidelines: evidence based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia 2006;47(7):1094‐120. - PubMed
Juul‐Jenson 1983
-
- Juul‐Jenson P, Foldspang A. Natural history of epileptic seizures. Epilepsia 1983;24(3):297‐312. - PubMed
Kirkham 2010
-
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. - PubMed
Kwan 2000
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314‐9. - PubMed
Lefebvre 2011
-
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Liporace 1994
-
- Liporace JD, Sperling MR, Dichter MA. Absence seizures and carbamazepine in adults. Epilepsia 1994;35(5):1026‐8. - PubMed
MacDonald 1995
-
- MacDonald RL, Kelly KM. Antiepileptic drug mechanisms of action. Epilepsia 1995;36(Suppl 2):S2‐12. - PubMed
MacDonald 2000
-
- MacDonald BK, Johnson AL, Goodridge DM, Cockerell OC, Sander JWA, Shorvon SD. Factors predicting prognosis of epilepsy after presentation with seizures. Annals of Neurology 2000;48(6):833‐41. - PubMed
Malafosse 1994
-
- Malfosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R. Idiopathic Generalised Epilepsies: Clinical, Experimental and Genetic. Eastleigh: John Libbey and Company, 1994.
Marson 2000
Matlow 2012
Meador 2008
Morrow 2006
Murray 1994
-
- Murray CJ, Lopez AD, World Health Organization. Global Comparative Assessments in the Health Sector: Disease Burden, Expenditures and Intervention Packages. Geneva: World Health Organization, 1994.
Nevitt 2017a
Nevitt 2017b
Nevitt 2018a
Nevitt 2018b
Nevitt 2018c
Nevitt 2018d
Ngugi 2010
NICE 2012
-
- National Institute for Health and Care Excellence. Epilepsies: diagnosis and management. Clinical guideline [CG137]. www.nice.org.uk/guidance/cg1373 (accessed 10 November 2016). London: National Institute for Health and Care Excellence, 2012.
Nolan 2013a
-
- Nolan SJ, Sutton L, Marson A, Tudur Smith C. Consistency of outcome and statistical reporting of time‐to‐event data: the impact on Cochrane Reviews and meta‐analyses in epilepsy. Better knowledge for better health. 21st Cochrane Colloquium; 19‐23 September 2013; Quebec City, Canada. Quebec City, Canada: The Cochrane Collaboration, 2013:114‐5.
Nolan 2013b
Olafsson 2005
-
- Olafsson E, Ludvigsson P, Gudmundsson G, Hesdorfer D, Kjartansson O, Hauser WA. Incidence of unprovoked seizures and epilepsy in Iceland and assessment of the epilepsy syndrome classification: a prospective study. Lancet Neurology 2005;4:627‐34. - PubMed
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. - PubMed
Ragsdale 1991
-
- Ragsdale DS, Scheuer T, Catterall WA. Frequency and voltage‐dependent inhibition of type IIA Na+ channels,expressed in a mammalian cell line, by local anaesthetic,antiarrhythmic, and anticonvulsant drugs. Molecular Pharmacology 1991;40(5):756‐65. - PubMed
Review Manager 2014 [Computer program]
-
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
SANAD B 2007
Sander 1996
Sander 2004
-
- Sander JW. The use of anti‐epileptic drugs ‐ principles and practice. Epilepsia 2004;45(Suppl 6):28‐34. - PubMed
Scheffer 2017
Shakir 1980
-
- Shakir RA. Sodium valproate, phenytoin and carbamazepine as sole anticonvulsants. The Place of Sodium Valproate in the Treatment of Epilepsy. London: Academic Press Inc and the Royal Society of Medicine, 1980:7‐16.
Shields 1983
-
- Shields WD, Saslow E. Myoclonic, atonic, and absence seizures following institution of carbamazepine therapy in children. Neurology 1983;33(11):1487‐9. - PubMed
Snead 1985
-
- Snead OC, Hosey LC. Exacerbation of seizures in children by carbamazepine. New England Journal of Medicine 1985;313(15):916‐21. - PubMed
Stata 2015 [Computer program]
-
- StataCorp. Stata. Version 14. College Station, TX, USA: StataCorp, 2015.
Tierney 2007
Tudur Smith 2007
Weston 2016
White 1997
-
- White HS, Brown SD, Woodhead JH, Skeen GA, Wolf HH. Topiramate enhances GABA‐mediated chloride flux and GABA‐evoked chloride currents in murine brain neurons and increases seizure threshold. Epilepsy Research 1997;28(3):167‐79. - PubMed
Williamson 2002
-
- Williamson PR, Tudur Smith C, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(11):3337‐51. - PubMed
References to other published versions of this review
Nolan 2016a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

