Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B
- PMID: 31233493
- PMCID: PMC6590788
- DOI: 10.1371/journal.pbio.3000311
Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B
Erratum in
-
Correction: Selection and characterization of ultrahigh potency designed ankyrin repeat protein inhibitors of C. difficile toxin B.PLoS Biol. 2019 Oct 4;17(10):e3000514. doi: 10.1371/journal.pbio.3000514. eCollection 2019 Oct. PLoS Biol. 2019. PMID: 31584930 Free PMC article.
Abstract
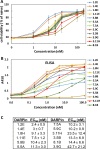
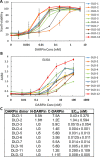
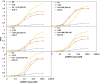
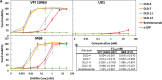
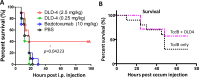
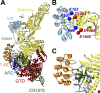
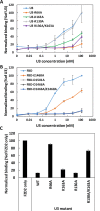
Clostridium difficile infection (CDI) is a major nosocomial disease associated with significant morbidity and mortality. The pathology of CDI stems primarily from the 2 C. difficile-secreted exotoxins-toxin A (TcdA) and toxin B (TcdB)-that disrupt the tight junctions between epithelial cells leading to the loss of colonic epithelial barrier function. Here, we report the engineering of a series of monomeric and dimeric designed ankyrin repeat proteins (DARPins) for the neutralization of TcdB. The best dimeric DARPin, DLD-4, inhibited TcdB with a half maximal effective concentration (EC50) of 4 pM in vitro, representing an approximately 330-fold higher potency than the Food and Drug Administration (FDA)-approved anti-TcdB monoclonal antibody bezlotoxumab in the same assay. DLD-4 also protected mice from a toxin challenge in vivo. Cryo-electron microscopy (cryo-EM) studies revealed that the 2 constituent DARPins of DLD-4-1.4E and U3-bind the central and C-terminal regions of the delivery domain of TcdB. Competitive enzyme-linked immunosorbent assay (ELISA) studies showed that the DARPins 1.4E and U3 interfere with the interaction between TcdB and its receptors chondroitin sulfate proteoglycan 4 (CSPG4) and frizzled class receptor 2 (FZD2), respectively. Our cryo-EM studies revealed a new conformation of TcdB (both apo- and DARPin-bound at pH 7.4) in which the combined repetitive oligopeptides (CROPS) domain points away from the delivery domain. This conformation of the CROPS domain is in stark contrast to that seen in the negative-stain electron microscopy (EM) structure of TcdA and TcdB at the same pH, in which the CROPS domain bends toward and "kisses" the delivery domain. The ultrapotent anti-TcdB molecules from this study serve as candidate starting points for CDI drug development and provide new biological tools for studying the pathogenicity of C. difficile. The structural insights regarding both the "native" conformation of TcdB and the putative sites of TcdB interaction with the FZD2 receptor, in particular, should help accelerate the development of next-generation anti-C. difficile toxin therapeutics.
Conflict of interest statement
The Texas A&M University and the University of Maryland have submitted a patent application on the anti-toxin DARPins reported in this study.
Figures



Similar articles
-
Designed Ankyrin Repeat Protein (DARPin) Neutralizers of TcdB from Clostridium difficile Ribotype 027.mSphere. 2019 Oct 2;4(5):e00596-19. doi: 10.1128/mSphere.00596-19. mSphere. 2019. PMID: 31578248 Free PMC article.
-
Functional defects in Clostridium difficile TcdB toxin uptake identify CSPG4 receptor-binding determinants.J Biol Chem. 2017 Oct 20;292(42):17290-17301. doi: 10.1074/jbc.M117.806687. Epub 2017 Aug 23. J Biol Chem. 2017. PMID: 28842504 Free PMC article.
-
Structural basis for CSPG4 as a receptor for TcdB and a therapeutic target in Clostridioides difficile infection.Nat Commun. 2021 Jun 18;12(1):3748. doi: 10.1038/s41467-021-23878-3. Nat Commun. 2021. PMID: 34145250 Free PMC article.
-
The Importance of Therapeutically Targeting the Binary Toxin from Clostridioides difficile.Int J Mol Sci. 2021 Mar 13;22(6):2926. doi: 10.3390/ijms22062926. Int J Mol Sci. 2021. PMID: 33805767 Free PMC article. Review.
-
Receptor binding mechanisms of Clostridioides difficile toxin B and implications for therapeutics development.FEBS J. 2023 Feb;290(4):962-969. doi: 10.1111/febs.16310. Epub 2021 Dec 13. FEBS J. 2023. PMID: 34862749 Free PMC article. Review.
Cited by
-
Molecular basis of TMPRSS2 recognition by Paeniclostridium sordellii hemorrhagic toxin.Nat Commun. 2024 Mar 4;15(1):1976. doi: 10.1038/s41467-024-46394-6. Nat Commun. 2024. PMID: 38438396 Free PMC article.
-
Recognition of Semaphorin Proteins by P. sordellii Lethal Toxin Reveals Principles of Receptor Specificity in Clostridial Toxins.Cell. 2020 Jul 23;182(2):345-356.e16. doi: 10.1016/j.cell.2020.06.005. Epub 2020 Jun 25. Cell. 2020. PMID: 32589945 Free PMC article.
-
Exotoxin-Targeted Drug Modalities as Antibiotic Alternatives.ACS Infect Dis. 2022 Mar 11;8(3):433-456. doi: 10.1021/acsinfecdis.1c00296. Epub 2022 Jan 31. ACS Infect Dis. 2022. PMID: 35099182 Free PMC article. Review.
-
A Multi-Specific DARPin Potently Neutralizes Shiga Toxin 2 via Simultaneous Modulation of Both Toxin Subunits.Bioengineering (Basel). 2022 Sep 27;9(10):511. doi: 10.3390/bioengineering9100511. Bioengineering (Basel). 2022. PMID: 36290479 Free PMC article.
-
Designed Ankyrin Repeat Protein (DARPin) Neutralizers of TcdB from Clostridium difficile Ribotype 027.mSphere. 2019 Oct 2;4(5):e00596-19. doi: 10.1128/mSphere.00596-19. mSphere. 2019. PMID: 31578248 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

