Biochemical characterization of the Helicobacter pylori bactofilin-homolog HP1542
- PMID: 31233532
- PMCID: PMC6590870
- DOI: 10.1371/journal.pone.0218474
Biochemical characterization of the Helicobacter pylori bactofilin-homolog HP1542
Abstract
The human pathogen Helicobacter pylori is known for its colonization of the upper digestive system, where it escapes the harsh acidic environment by hiding in the mucus layer. One factor promoting this colonization is the helical cell shape of H. pylori. Among shape determining proteins are cytoskeletal elements like the recently discovered bactofilins. Bactofilins constitute a widespread family of polymer-forming bacterial proteins whose biology is still poorly investigated. Here we describe the first biochemical analysis of the bactofilin HP1542 of H. pylori reference strain 26695. Purified HP1542 forms sheet-like 2D crystalline assemblies, which clearly depend on a natively structured C-terminus. Polymerization properties and protein stability were investigated. Additionally, we also could demarcate HP1542 from amyloid proteins that share similarities with the bactofilin DUF domain. By using zonal centrifugation of total H. pylori cell lysates and immunfluorescence analysis we revealed peripheral membrane association of HP1542 mostly pronounced near mid-cell. Interestingly our results indicate that H. pylori bactofilin does not contribute to cell wall stability. This study might act as a starting point for biophysical studies of the H. pylori bactofilin biology as well as for the investigation of bactofilin cell physiology in this organism. Importantly, this study is the first biochemical analysis of a bactofilin in a human pathogen.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

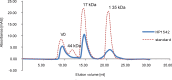
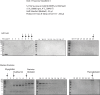

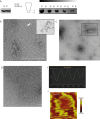

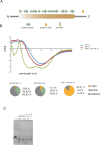

References
-
- Cho H. The role of cytoskeletal elements in shaping bacterial cells. Journal of microbiology and biotechnology. 2015;25(3):307–16. Epub 2014/09/30. . - PubMed
-
- Vasa S, Lin L, Shi C, Habenstein B, Riedel D, Kuhn J, et al. beta-Helical architecture of cytoskeletal bactofilin filaments revealed by solid-state NMR. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(2):E127–36. Epub 2015/01/01. 10.1073/pnas.1418450112 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

