Reciprocal signaling and direct physical interactions between fibroblasts and breast cancer cells in a 3D environment
- PMID: 31233557
- PMCID: PMC6590889
- DOI: 10.1371/journal.pone.0218854
Reciprocal signaling and direct physical interactions between fibroblasts and breast cancer cells in a 3D environment
Abstract
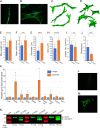
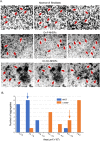
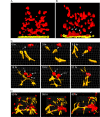
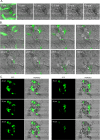
Tumorigenic cells undergo cell aggregation and aggregate coalescence in a 3D Matrigel environment. Here, we expanded this 3D platform to assess the interactions of normal human dermal fibroblasts (NHDFs) and human primary mammary fibroblasts (HPMFs) with breast cancer-derived, tumorigenic cells (MDA-MB-231). Medium conditioned by MDA-MB-231 cells activates both types of fibroblasts, imbuing them with the capacity to accelerate the rate of aggregation and coalescence of MDA-MB-231 cells more than four fold. Acceleration is achieved 1) by direct physical interactions with MDA-MB-231 cells, in which activated fibroblasts penetrate the MDA-MB-231/Matrigel 3D environment and function as supporting scaffolds for MDA-MB-231 aggregation and coalescence, and 2) through the release of soluble accelerating factors, including matrix metalloproteinase (MMPs) and, in the case of activated NHDFs, SDF-1α/CXCL12. Fibroblast activation includes changes in morphology, motility, and gene expression. Podoplanin (PDPN) and fibroblast activation protein (FAP) are upregulated by more than nine-fold in activated NHDFs while activated HPMFs upregulate FAP, vimentin, desmin, platelet derived growth factor receptor A and S100A4. Overexpression of PDPN, but not FAP, in NHDF cells in the absence of MDA-MB-231-conditioned medium, activates NHDFs. These results reveal that complex reciprocal signaling between fibroblasts and cancer cells, coupled with their physical interactions, occurs in a highly coordinated fashion that orchestrates aggregation and coalescence, behaviors specific to cancer cells in a 3D environment. These in vitro interactions may reflect events involved in early tumorigenesis, particularly in cases of field cancerization, and may represent a new mechanism whereby cancer-associated fibroblasts (CAFs) promote tumor growth.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Carcinoma associated fibroblasts (CAFs) promote breast cancer motility by suppressing mammalian Diaphanous-related formin-2 (mDia2).PLoS One. 2018 Mar 29;13(3):e0195278. doi: 10.1371/journal.pone.0195278. eCollection 2018. PLoS One. 2018. PMID: 29596520 Free PMC article.
-
Fibroblast growth factor-2, derived from cancer-associated fibroblasts, stimulates growth and progression of human breast cancer cells via FGFR1 signaling.Mol Carcinog. 2020 Sep;59(9):1028-1040. doi: 10.1002/mc.23233. Epub 2020 Jun 18. Mol Carcinog. 2020. PMID: 32557854
-
Tumor hypoxia modulates podoplanin/CCL21 interactions in CCR7+ NK cell recruitment and CCR7+ tumor cell mobilization.Oncotarget. 2017 May 9;8(19):31876-31887. doi: 10.18632/oncotarget.16311. Oncotarget. 2017. PMID: 28416768 Free PMC article.
-
The fibroblastic coconspirator in cancer progression.Cold Spring Harb Symp Quant Biol. 2005;70:383-8. doi: 10.1101/sqb.2005.70.007. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869775 Free PMC article. Review.
-
Progress towards understanding heterotypic interactions in multi-culture models of breast cancer.Integr Biol (Camb). 2016 Jun 13;8(6):684-92. doi: 10.1039/c6ib00001k. Epub 2016 Apr 21. Integr Biol (Camb). 2016. PMID: 27097801 Free PMC article. Review.
Cited by
-
Modelling the complex nature of the tumor microenvironment: 3D tumor spheroids as an evolving tool.J Biomed Sci. 2024 Jan 23;31(1):13. doi: 10.1186/s12929-024-00997-9. J Biomed Sci. 2024. PMID: 38254117 Free PMC article. Review.
-
3D and 4D Tumorigenesis Model for the Quantitative Analysis of Cancer Cell Behavior and Screening for Anticancer Drugs.Methods Mol Biol. 2022;2364:299-318. doi: 10.1007/978-1-0716-1661-1_14. Methods Mol Biol. 2022. PMID: 34542859
-
Dermal fibroblasts and triple-negative mammary epithelial cancer cells differentially stiffen their local matrix.APL Bioeng. 2020 Dec 4;4(4):046105. doi: 10.1063/5.0021030. eCollection 2020 Dec. APL Bioeng. 2020. PMID: 33305163 Free PMC article.
-
Morphological and Molecular Changes in Juvenile Normal Human Fibroblasts Exposed to Simulated Microgravity.Sci Rep. 2019 Aug 15;9(1):11882. doi: 10.1038/s41598-019-48378-9. Sci Rep. 2019. PMID: 31417174 Free PMC article.
-
In vitro detection of cancer cells using a novel fluorescent choline derivative.BMC Med Imaging. 2024 Nov 20;24(1):316. doi: 10.1186/s12880-024-01488-x. BMC Med Imaging. 2024. PMID: 39567942 Free PMC article.
References
-
- Liakou E, Mavrogonatou E, Pratsinis H, Rizou S, Evangelou K, Panagiotou PN, et al. Ionizing radiation-mediated premature senescence and paracrine interactions with cancer cells enhance the expression of syndecan 1 in human breast stromal fibroblasts: the role of TGF-beta. Aging. 2016;8(8):1650–69. Epub 2016/07/20. 10.18632/aging.100989 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

