Glutamate receptor interacting protein acts within the prefrontal cortex to blunt cocaine seeking
- PMID: 31233823
- PMCID: PMC6709847
- DOI: 10.1016/j.neuropharm.2019.107672
Glutamate receptor interacting protein acts within the prefrontal cortex to blunt cocaine seeking
Abstract
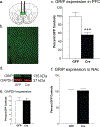
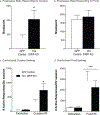
Glutamate receptor interacting protein (GRIP) is a neuronal scaffolding protein that anchors GluA2-containing AMPA receptors to the cell membrane. GRIP plays a critical role in activity-dependent synaptic plasticity, including that which occurs after drug exposure. Given that cocaine administration alters glutamate receptor trafficking within the prefrontal cortex (PFC), a better understanding of the role of receptor trafficking proteins could lead to a more complete understanding of addictive phenotypes. AMPA receptor trafficking in general, and GRIP specifically, is known to play a role in cocaine seeking and conditioned reward in the nucleus accumbens, but its role in the PFC has not been characterized. The current study demonstrates that conditional deletion of GRIP1 in the medial prefrontal cortex increases the motivation for cocaine and potentiates cue-induced reinstatement of cocaine seeking in male and female mice. As no effects of PFC GRIP1 deletion were seen in reinstatement of food seeking, strategy set-shifting, or reversal learning the effects on cocaine seeking are not related to generalized alterations in cognitive function. While disrupting GRIP1 might be expected to lead to decreased AMPA transmission, our electrophysiological data indicate an increase in sEPSC amplitude in the prefrontal cortex and a corresponding decrease in paired pulse facilitation in the nucleus accumbens. Taken together this suggests a strengthening of the PFC to NAc input following prefrontal GRIP1 deletion that may mediate the enhanced drug seeking behavior.
Keywords: AMPA; Cocaine; Glutamate receptor interacting protein; Prefrontal cortex; Reinstatement.
Copyright © 2019 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Disclosure
The authors declare no conflict of interest.
Figures

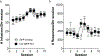

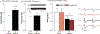
Similar articles
-
Disruption of glutamate receptor-interacting protein in nucleus accumbens enhances vulnerability to cocaine relapse.Neuropsychopharmacology. 2014 Feb;39(3):759-69. doi: 10.1038/npp.2013.265. Epub 2013 Oct 15. Neuropsychopharmacology. 2014. PMID: 24126453 Free PMC article.
-
Disrupting GluA2 phosphorylation potentiates reinstatement of cocaine seeking.Neuropharmacology. 2016 Dec;111:231-241. doi: 10.1016/j.neuropharm.2016.09.010. Epub 2016 Sep 10. Neuropharmacology. 2016. PMID: 27622930 Free PMC article.
-
Persistent strengthening of the prefrontal cortex - nucleus accumbens pathway during incubation of cocaine-seeking behavior.Neurobiol Learn Mem. 2017 Feb;138:281-290. doi: 10.1016/j.nlm.2016.10.003. Epub 2016 Oct 5. Neurobiol Learn Mem. 2017. PMID: 27720809
-
Differential roles of medial prefrontal subregions in the regulation of drug seeking.Brain Res. 2015 Dec 2;1628(Pt A):130-46. doi: 10.1016/j.brainres.2014.12.024. Epub 2014 Dec 18. Brain Res. 2015. PMID: 25529632 Free PMC article. Review.
-
Infralimbic cortex functioning across motivated behaviors: Can the differences be reconciled?Neurosci Biobehav Rev. 2021 Dec;131:704-721. doi: 10.1016/j.neubiorev.2021.10.002. Epub 2021 Oct 5. Neurosci Biobehav Rev. 2021. PMID: 34624366 Free PMC article. Review.
Cited by
-
Glutamate receptor-interacting protein 1 in D1- and D2-dopamine receptor-expressing medium spiny neurons differentially regulates cocaine acquisition, reinstatement, and associated spine plasticity.Front Cell Neurosci. 2022 Nov 3;16:979078. doi: 10.3389/fncel.2022.979078. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36406750 Free PMC article.
-
Esketamine Inhibits Cocaine-Seeking Behaviour Subsequent to Various Abstinence Conditions in Rats.Biomolecules. 2023 Sep 19;13(9):1411. doi: 10.3390/biom13091411. Biomolecules. 2023. PMID: 37759811 Free PMC article.
-
Circulating ovarian hormones interact with protein interacting with C kinase (PICK1) within the medial prefrontal cortex to influence cocaine seeking in female mice.Horm Behav. 2023 Sep;155:105408. doi: 10.1016/j.yhbeh.2023.105408. Epub 2023 Aug 2. Horm Behav. 2023. PMID: 37541099 Free PMC article.
-
Sex-specific role for prefrontal cortical protein interacting with C kinase 1 in cue-induced cocaine seeking.Addict Biol. 2021 Sep;26(5):e13051. doi: 10.1111/adb.13051. Epub 2021 Jun 10. Addict Biol. 2021. PMID: 34110073 Free PMC article.
-
PKMζ alters oxycodone-taking in a dose- and sex-dependent manner.Addict Neurosci. 2024 Sep;12:100169. doi: 10.1016/j.addicn.2024.100169. Epub 2024 Jul 29. Addict Neurosci. 2024. PMID: 39449991 Free PMC article.
References
-
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC, 2008. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci 11, 344–353. - PubMed
-
- Bechara A, Tranel D, Damasio H, 2000. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123 ( Pt 11), 2189–2202. - PubMed
-
- Ben-Shahar OM, Szumlinski KK, Lominac KD, Cohen A, Gordon E, Ploense KL, DeMartini J, Bernstein N, Rudy NM, Nabhan AN, Sacramento A, Pagano K, Carosso GA, Woodward N, 2012. Extended access to cocaine self-administration results in reduced glutamate function within the medial prefrontal cortex. Addiction biology 17, 746–757. - PMC - PubMed
-
- Berke JD, Hyman SE, 2000. Addiction, dopamine, and the molecular mechanisms of memory. Neuron 25, 515–532. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

