Lysosome-related organelles as functional adaptations of the endolysosomal system
- PMID: 31234051
- PMCID: PMC6726539
- DOI: 10.1016/j.ceb.2019.05.003
Lysosome-related organelles as functional adaptations of the endolysosomal system
Erratum in
-
Corrigendum to "Lysosome related organelles as functional adaptations of the endolysosomal system", Current Opinion in Cell Biology, volume 59, 2019, 147-158.Curr Opin Cell Biol. 2019 Dec;61:141. doi: 10.1016/j.ceb.2019.11.003. Curr Opin Cell Biol. 2019. PMID: 31831109 No abstract available.
Abstract
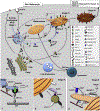
Unique functions of specialised cells such as those of the immune and haemostasis systems, skin, blood vessels, lung, and bone require specialised compartments, collectively referred to as lysosome-related organelles (LROs), that share features of endosomes and lysosomes. LROs harbour unique morphological features and cell type-specific contents, and most if not all undergo regulated secretion for diverse functions. Ongoing research, largely driven by analyses of inherited diseases and their model systems, is unravelling the mechanisms involved in LRO generation, maturation, transport and secretion. A molecular understanding of these features will provide targets and markers that can be exploited for diagnosis and therapy of a myriad of diseases.
Copyright © 2019. Published by Elsevier Ltd.
Figures


Similar articles
-
Lysosome-related organelles: unusual compartments become mainstream.Curr Opin Cell Biol. 2013 Aug;25(4):495-505. doi: 10.1016/j.ceb.2013.04.008. Epub 2013 May 29. Curr Opin Cell Biol. 2013. PMID: 23726022 Free PMC article. Review.
-
Lysosome-related organelles: driving post-Golgi compartments into specialisation.Curr Opin Cell Biol. 2007 Aug;19(4):394-401. doi: 10.1016/j.ceb.2007.05.001. Epub 2007 Jul 12. Curr Opin Cell Biol. 2007. PMID: 17628466 Free PMC article. Review.
-
An endosomal syntaxin and the AP-3 complex are required for formation and maturation of candidate lysosome-related secretory organelles (mucocysts) in Tetrahymena thermophila.Mol Biol Cell. 2017 Jun 1;28(11):1551-1564. doi: 10.1091/mbc.E17-01-0018. Epub 2017 Apr 5. Mol Biol Cell. 2017. PMID: 28381425 Free PMC article.
-
Endolysosomal Cation Channels and Lung Disease.Cells. 2022 Jan 17;11(2):304. doi: 10.3390/cells11020304. Cells. 2022. PMID: 35053420 Free PMC article. Review.
-
Linking exocytosis and endocytosis during phagocytosis.Biol Cell. 2006 Mar;98(3):195-201. doi: 10.1042/BC20050021. Biol Cell. 2006. PMID: 16480341
Cited by
-
PKA Activity-Driven Modulation of Bidirectional Long-Distance transport of Lysosomal vesicles During Synapse Maintenance.bioRxiv [Preprint]. 2024 Jun 29:2024.06.28.601272. doi: 10.1101/2024.06.28.601272. bioRxiv. 2024. PMID: 38979384 Free PMC article. Preprint.
-
Melanocytes in regenerative medicine applications and disease modeling.J Transl Med. 2024 Apr 8;22(1):336. doi: 10.1186/s12967-024-05113-x. J Transl Med. 2024. PMID: 38589876 Free PMC article. Review.
-
Morphodynamical adaptation of the endolysosomal system to stress.FEBS J. 2025 Jan;292(2):248-260. doi: 10.1111/febs.17154. Epub 2024 May 5. FEBS J. 2025. PMID: 38706230 Free PMC article. Review.
-
The Small GTPase Rab7 Regulates Antigen Processing in B Cells in a Possible Interplay with Autophagy Machinery.Cells. 2023 Nov 2;12(21):2566. doi: 10.3390/cells12212566. Cells. 2023. PMID: 37947644 Free PMC article.
-
Biogenesis of specialized lysosomes in differentiated keratinocytes relies on close apposition with the Golgi apparatus.Cell Death Dis. 2024 Jul 11;15(7):496. doi: 10.1038/s41419-024-06710-w. Cell Death Dis. 2024. PMID: 38992005 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

